Build Your Own Potency Assay
using
STEMGlo™ SC-IPS
A Basic Standardized and Validated Bioluminescence Cell Potency Starter Assay Platform
Buy STEMGlo™ SC-IPS
STEMGlo™ SC-IPS
A Standardized and Validated In Vitro Bioluminescence Assay to Measure Potency and Quality of Stem Cell Lines
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| ES, iPS and other stem cell lines (adherent) | Any | User defined | KSG-PQRA-1 | 1 Kit |
| ES, iPS and other stem cell lines (non‑adherent) | Any | User defined | KSG-PQRNA-1 | 1 Kit |
What is Potency?
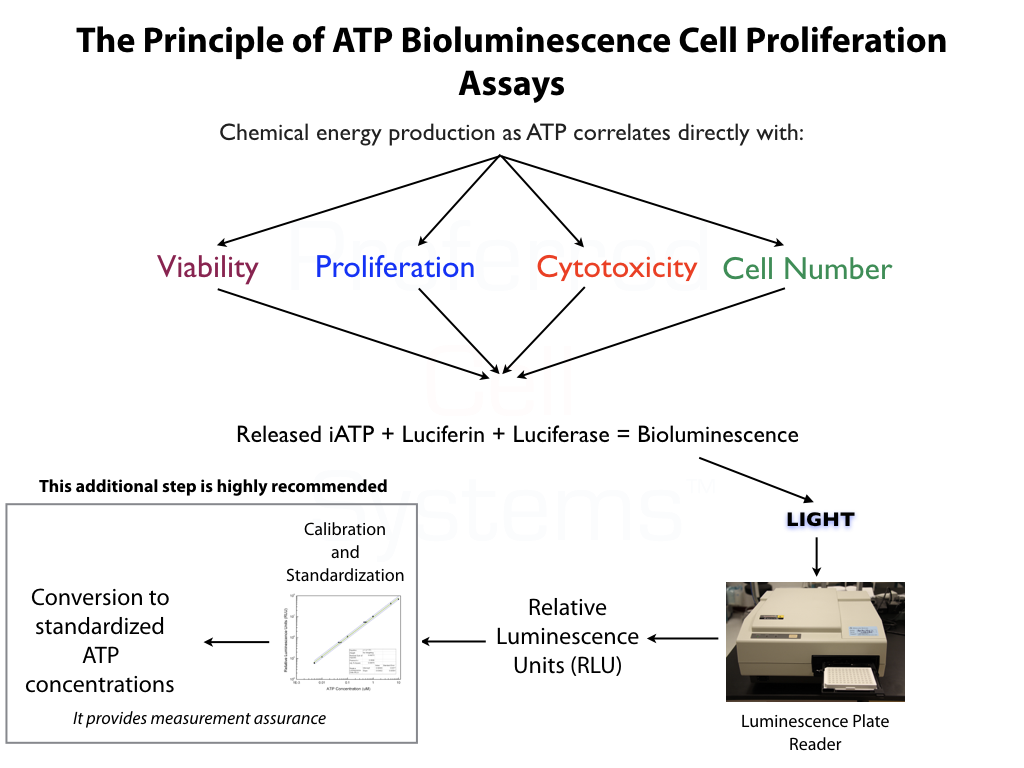
- Cell potency is the quantitative and validated measurement of proliferation potential or capacity (biological activity) of the "active" cell components to produce the intended effect or response.
- Cell proliferation potential or capacity correlates directly with the primitiveness of the cells in question. For stem cells, the greater their proliferation potential, the greater their self-renewal capacity and the more primitive the stem cell population. The more primitive a cell population, the greater its potency.
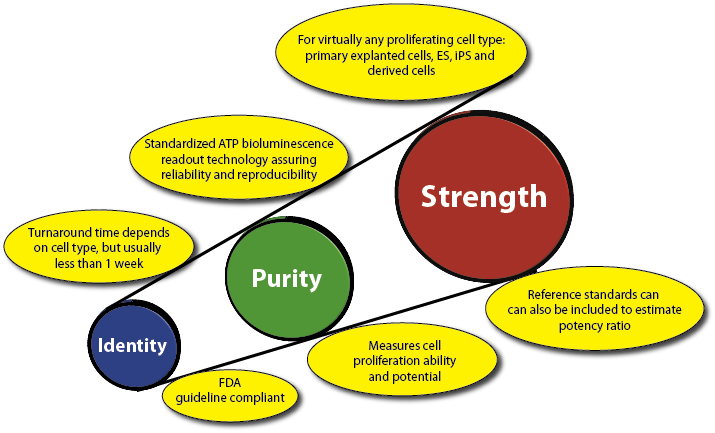
- STEMGlo™ SC-IPS can be used to determines cell potency and quality in a single assay for virtually any proliferating cell product used in a clinical setting.
- STEMGlo™ SC-IPS determines the identity, purity and strength or potency of a cell population compared to either a reference standard or similar cell source.
- The assay kit also allows for a reference standard (RS) to be established to determine the potency ratio.
- STEMGlo™ SC-IPS allows promotion of "Best Practice Criteria Testing."
- Allows determination of cell identity, purity and strength of a cell population or source if compared to a standard source.
- Allows determination of the cell potency ratio and cell quality simultaneously.
- Uses the standardized 'Slope-Ratio Concentration-Response Model" for measuring potency as described in the U.S. Pharmacopeia.
- Instrument-based, incorporating an external standard and controls for assay calibration, standardization and validation according to FDA guidelines.
- Allows for intra- and inter-laboratory comparisons.
- Establish all the parameters needed to apply the assay as more biological functional chacracteristics accrue.
- Can help any laboratory achieve "Best Practices" and measurement assurance promoted by the National Institute of Standards and Technology (NIST) .
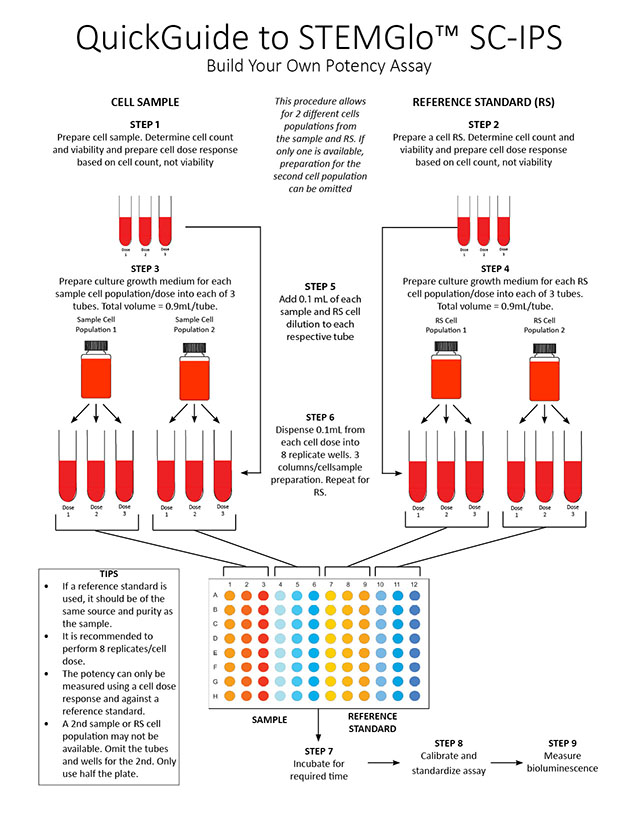
- To measure cell strength or potency, a reference standard (RS) or a cell source of the same material and purity as the sample is usually required so that the potency ratio (the measure of potency) can be determined. The cell sample is directly compared to the standard to determine if the cell sample has the same identity, purity and strength.
- STEMGlo™ IPS can be used to establish a reference standard for a specific cell type. A batch of cells is prepared, processed, aliquoted and cryopreserved and it's strength determined. Other secondary and tertiary RSs are also prepared. The RS is then used to compare to the sample of unknown strength.
- Any proliferating cell type or cells with high intracellular ATP can be used.
- The greater the purity, the more accurate the potency determination. However, the purity should be similar to that of the clinical sample.
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader capable of measuring "glow" luminescence is needed for this assay.
- Base medium for diluting ATP standard
- ATP standards
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plate(s)
- Non-sterile, 96-well plate(s)
- Sterile, adhesive foil covers