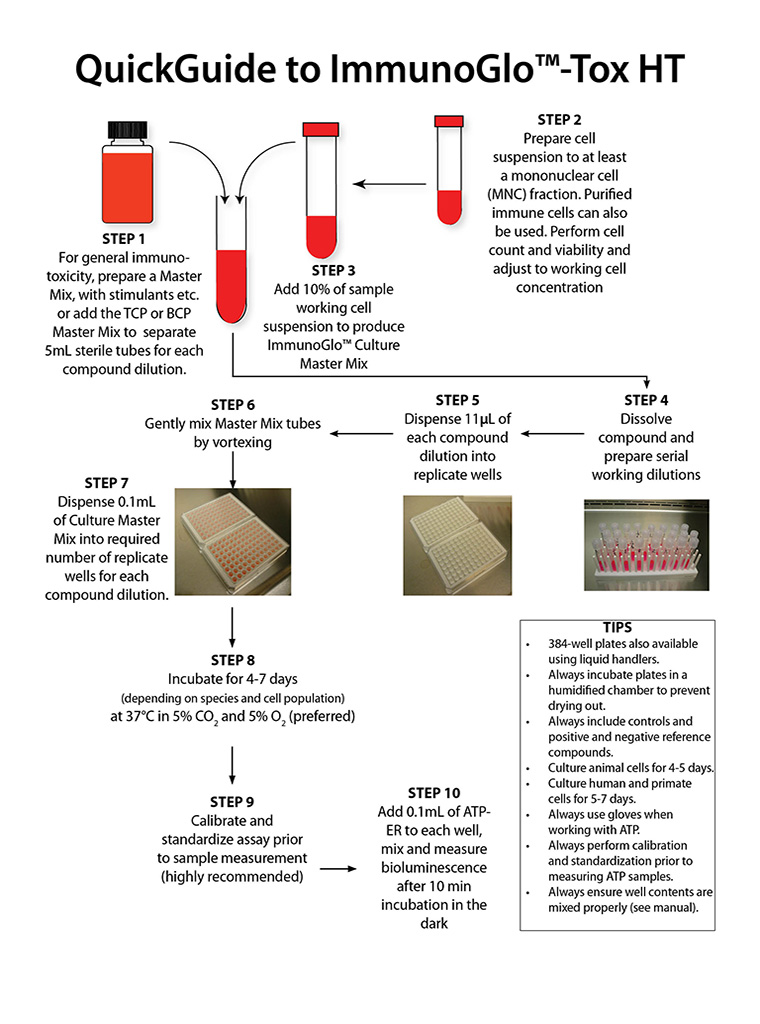
ImmunoGlo™-Tox HT
An In Vitro Standardized and Validated
ATP Bioluminescence Immunotoxicity
Testing and High-Throughput Screening Platform
Buy ImmunoGlo™-Tox HT
ImmunoGlo™-Tox HT
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Immune cells | Multiple / Any | ImmunoGro™ Base Medium | KM1-T96-4 | 1 Kit |
ImmunoGlo™-Tox HT TCP
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species/Tissue | ImmunoGro™ Medium Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Low Serum | |||||
| T-lymphocytes | Human / Any | Low Serum + IL-2 | KM1-T96CS1-4H | 1 Kit | |
| T-lymphocytes | Human / Any | Low Serum + CD3 + CD28 | KM1-T96CS2-4H | 1 Kit | |
| T-lymphocytes | Human / Any | Low Serum + IL-2 + CD3 + CD28 | KM1-T96CS3-4H | 1 Kit | |
| Serum Free | |||||
| T-lymphocytes | Human / Any | Serum-free + IL-2 | KM1SF-T96CS1-4H | 1 Kit | |
| T-lymphocytes | Human / Any | Serum-free + CD3 + CD28 | KM1SF-T96CS2-4H | 1 Kit | |
| T-lymphocytes | Human / Any | Serum-free + IL-2 + CD3 + CD28 | KM1SF-T96CS3-4H | 1 Kit | |
ImmunoGlo™ is trademark by Preferred Cell Systems
ImmunoGlo™-Tox HT TCP
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species/Tissue | ImmunoGro™ Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| T-lymphocytes | Primate / Any | Low serum | KM1-T96CS1-4Pr | 1 Kit |
ImmunoGlo™ is trademark by Preferred Cell Systems
ImmunoGlo™-Tox HT TCP
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species/Tissue | ImmunoGro™ Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| T-lymphocytes | Rat / Any | Low serum | KM1-T96CS1-4R | 1 Kit |
ImmunoGlo™ is trademark by Preferred Cell Systems
ImmunoGlo™-Tox HT TCP
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species/Tissue | ImmunoGro™ Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| T-lymphocytes | Mouse / Any | Low serum | KM1-T96CS1-4M | 1 Kit |
ImmunoGlo™ is trademark by Preferred Cell Systems
ImmunoGlo™-Tox HT BCP
A Standardized and Validated In Vitro Bioluminescence Immunotoxicity Platform for T‑Lymphocytes
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| B-lymphocytes | Human / Any | Low Serum +Ligands+IL4 | KM1-B96C2-4H | 1 Kit |
| B-lymphocytes | Human / Any | Low Serum+Conjugates+Ligands+IL-4 | KM1-B96C3-4H | 1 Kit |

The ImmunoGlo™-Tox HT Family of Assays
- ImmunoGlo™-Tox HT is a general use functional immune cell proliferation assay where the investigator employs their own culture reagents and protocol for any type of immune cell.
- ImmunoGlo™ Tox HT TCP Assay Kits include specific cocktails to stimulate functional T-lymphocytes.
- ImmunoGlo™ Tox HT BCP is specific for stimulating functional human B-lymphocytes.
- In vitro high-throughput immunotoxicity screening.
- In vitro high throughput lymphocyte proliferation screening.
- Specialized toxicity assays for T- and B-lymphocytes.
- Cellular immune response screening.
- Development of drugs and immunotherapies using gene targeting, e.g. CRISPR-Cas9.
- Non-radioactive assay incorporating proven high-throughput ATP bioluminescence technology.
- Instrument-based, quantitative and fully standardized allowing comparison of results over time.
- Can be validated for GLP studies.
- More sensitive than WST-1, CSFE and other absorbance or fluorescence assays.
- Available in 96- or 384-well plate formats.
- Multiplexing capability to incorporate flow cytometry, cytokine release and other assay readouts for additional information from the same sample.
- Simple, time efficient and cost effective.
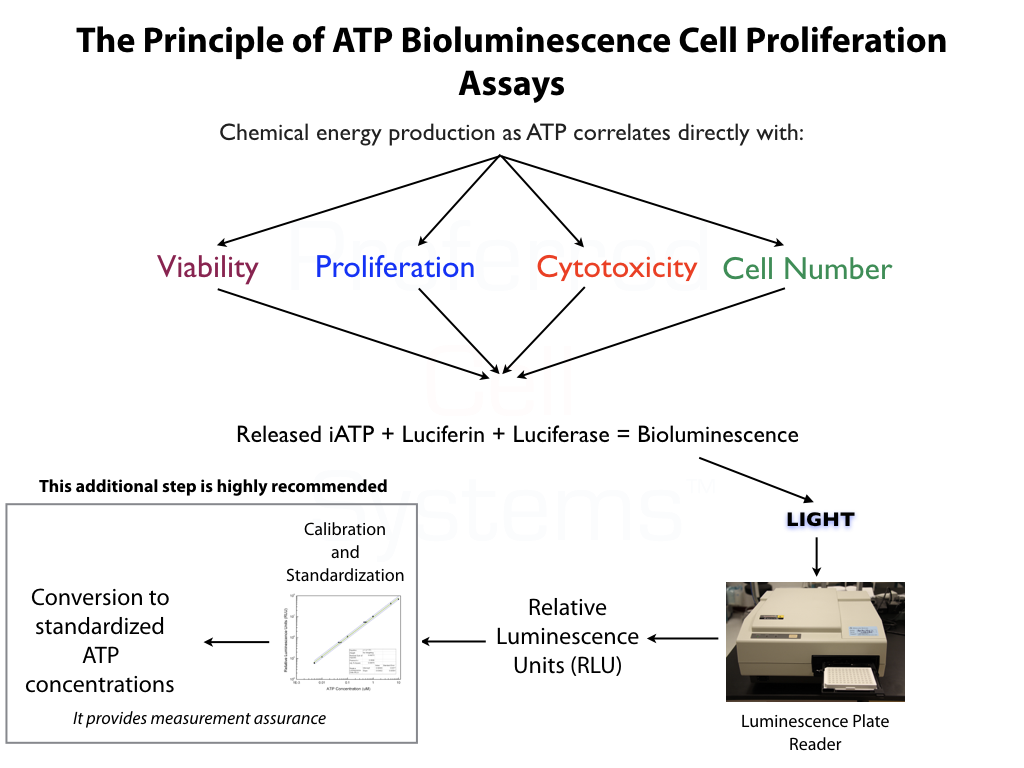
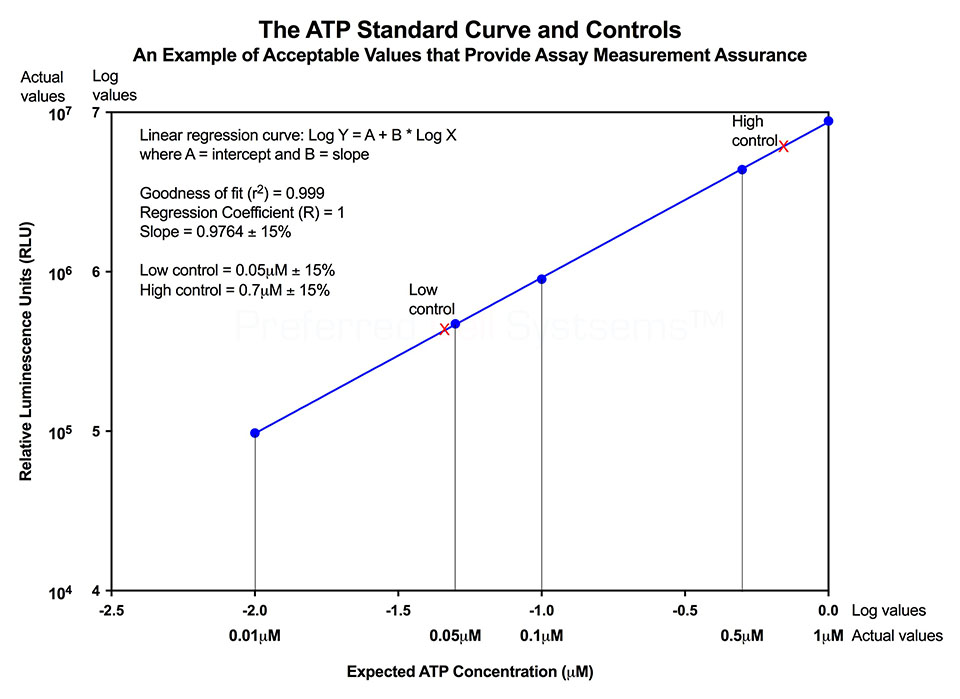
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- Human
- Primate
- Rat
- Mouse
- Peripheral blood mononuclear cells (PBMC)
- Primary lymphocyte sub-populations
- Primary organs and tissues containing immune cells
- Lymphocyte cell lines
For Research Use Only.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- Base medium for ATP standard dilution
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96- or 384-well culture plates
- Non-sterile, 96- or 384-well plates
- ImmunoGlo™ TCPMaster Mix for requested T-lymphocyte stimulation
- Base medium for ATP standard dilution
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96- or 384-well culture plates
- Non-sterile, 96- or 384-well plates
- ImmunoGlo™ BCP Master Mix for requested B-lymphocyte stimulation
- Base medium for ATP standard dilution
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96- or 384-well culture plates
- Non-sterile, 96- or 384-well plates
Download the ATP Optimization Kit Protocol for First-Time Users
Download Luminometer Setup and RLU to ATP Conversion
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM