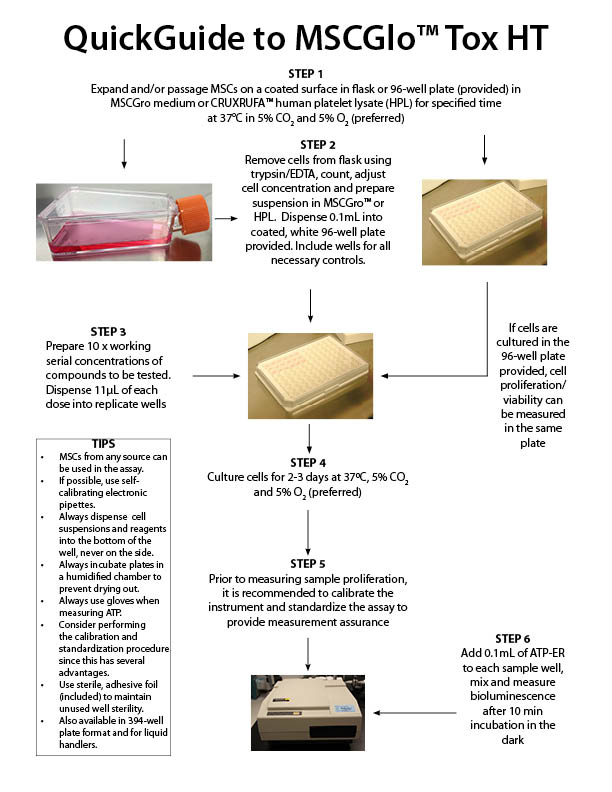
MSCGlo™-Tox HT
An In Vitro Standardized and Validated
ATP Bioluminescence Mesenchymal Cell Toxicity
Testing and High-Throughput Screening Platform
Buy MSCGlo™-Tox HT
MSCGlo™-Tox HT
A Standardized and Validated In Vitro Bioluminescence Assay to Detect Toxicity to Mesenchymal Cells (MSC)
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Human | Serum-free, Humanized | KLMC-T96SF-4 | 4 plate/1 Kit |

- Predictive in vitro to in vivo surrogate assay for human and animal MSC/MPC toxicity.
- Predictive in vitro MSC/MPC screening and testing platform for all stages of drug development.
- Predictive in vitro MSC/MPC toxicity screening and testing for xenobiotic agents.
- Incorporation into routine high throughput ADME/Tox screening.
- Drug/ compound ranking according to species and/or cell population sensitivity.
- Regenerative drug therapy using gene targeting methodology, e.g. CRISPR-Cas9.
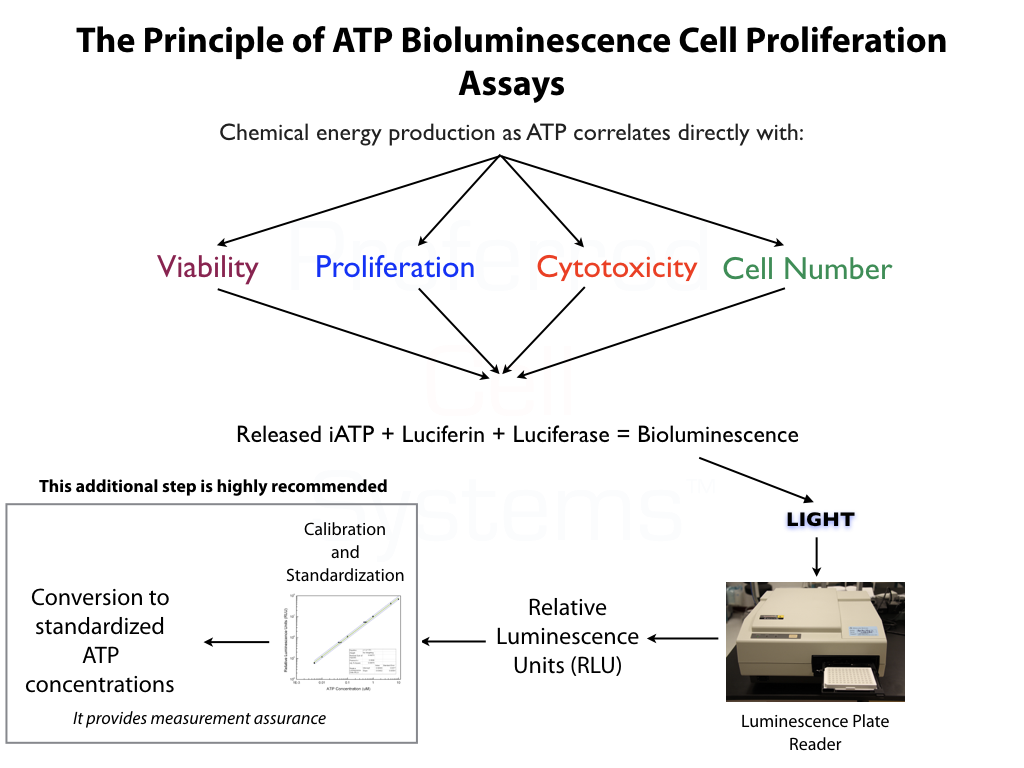
- High throughput, ATP bioluminescence assay to measure potential toxicity to the MSC/MPC system.
- Fully standardized with validation capability.
- A 3Rs Alternative Assay Platform for Reduction, Refinement and Replacement of animal testing.
- The only in vitro high throughput MSC toxicity assay platform available with both 96-well and 384-well plate formats.
- Rapid turnaround: results in about 3 days.
- Customized assay kits with either CRUXRUFA Human Platelet Lysate or different formulations of MSCGro™ media.
- Designed for multiplexing with other assays to produce the most amount of information from a single sample.
- Simple, time efficient and cost effective.
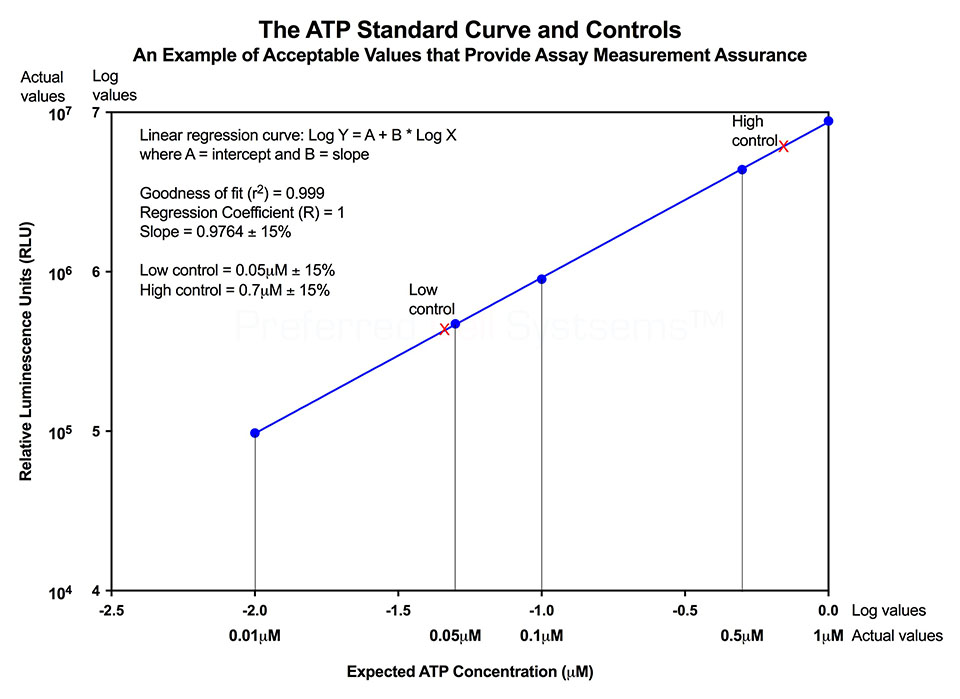
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- Human
- Non-human primate (Cynomologus and Rhesus)
- Rat
- Mouse
MSCGlo™-Tox HT can be used with MSCs derived from fresh primary tissues such as bone marrow and umbilical cord blood or MSCs derived from other cells such as induced pluripotent stem cells (iPSC).
For Research Use Only.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- MSCGro™ Medium of choice
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
When ordering MSCGlo™-Tox HT, click on the catalog number link in the table below to go directly to the product order page.
MSCGlo™-Tox HT Assay Kits
| Catalog Number | Species | MSC Growth Medium Formulation |
|---|---|---|
| KLMC-T96SF-2 | Human, Primate, Rat, Mouse | 3 |
| KLMC-T96SF-4 | Human, Primate, Rat, Mouse | Human |