HALO®: The Ultimate in Lympho-Hematopoietic Assays.
The HALO® Page
The Most Advanced Lympho-Hematopoietic Assay Platform for Discerning Scientists
HALO® is the acronym for Hematopoietic/Hematotoxicity Assays via Luminescence Output. HALO® is the flagship assay platform from which all other standardized ATP bioluminescence assays for different biological systems were derived.
HALO® is the most technologically advanced and sophisticated assay ever developed to detect and quantitatively measure the viability and proliferation / cytotoxicity of lympho-hematopoietic stem and progenitor cells Indeed, it is the only assay platform that demonstrates the following characteristics:
- Instrument-based.
- Non-subjective.
- Standardized.
- The assay is validated and complies with FDA Bioanalytical Method Validation Guidelines.
- Incorporates measurement assurance parameters to ensure the assay is working correctly and that results can be trusted.
- Proficiency testing is built into the assay.
- Uses Suspension Expansion Culture™ (SEC™) Technology and is, therefore, methylcellulose-free.
- The assay has high-throughput capability and can be used with 96- or 384-well plates using liquid handlers.
- Cell incubation is less than half the time of the colony-forming unit (CFU) assay (see below).
HALO® was completely rebuilt from the ground up from the traditional colony-forming unit (CFU) or cell (CFC) assay, first published in 1966. The First Generation of the assay used methylcellulose to grow colonies, but included an ATP bioluminescence signal detection system to measure proliferation/cytotoxicity in the colonies. This Second Generation Assay incorporated the calibration and standardization procedures used in HALO® and is now called CAMEO™-96. CAMEO™-96 remains, to this day, the only true standardized CFU assay available (see the diagram below).
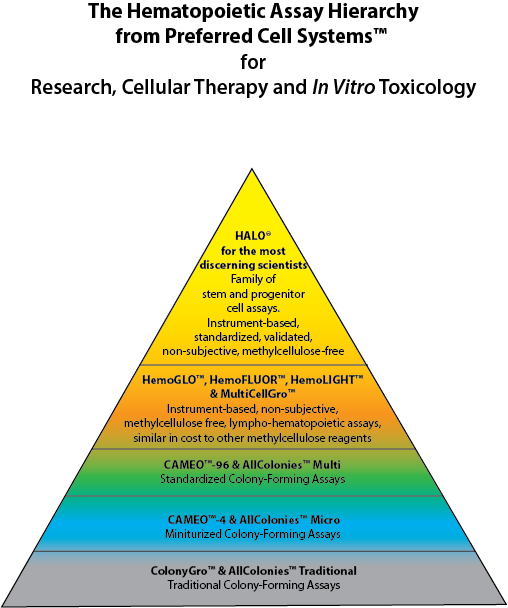
With the development of Suspension Expansion Culture™ (SEC™) Technology that obviated the use of methylcellulose, CAMEO™-96 became the HALO® Platform. The incorporation of SEC™ Technology coupled with the biochemical measurement of intracellular ATP (iATP) using luciferin/luciferase bioluminescence technology, allowed the transformation of the traditional CFU assay into what is now the most technologically advanced assay of its kind for the lympho-hematopoietic system.
Since it was launched in 2002, the HALO® Platform has been developed for human, non-human primate, horse, sheep, pig, minipig, dog, rat and mouse cells. It replaces the CFU assay for all applications in basic and stem cell research, cellular therapy and in vitro hematotoxicity testing. It has also been employed by half of the top 50 biopharmaceutical companies.
Unlike to CFU assay, and thanks to the incorporation of the most sensitive, non-radioactive signal detection system available, namely ATP bioluminescence, HALO® is capable of detecting the rarest of primitive lympho-hematopoietic stem cell populations.
Gone, are all the disadvantages and flaws of the CFU assay, such as dispensing errors, subjectivity, lack of reproducibility, inability to standardize and validate the assay, no measurement assurance parameters and lack of high-throughput and multitasking capability.
HALO® is at the apex of the Hematopoietic Assay Hierarchy. For those who do not need to perform a standardized or validated assay, Preferred Cell Systems™ has introduced HemoGLO™, which also employs an ATP bioluminescence readout. HemoGLO™, together with HemoFLUOR™ and HemoLIGHT™ provide the next layer of instrument-based, rapid to learn and easy to use assays that can be multiplexed with other readouts, such as flow cytometry.
The HALO® family of assays consists of the following:
1. Stem Cell and Basic Hematopoietic Research Applications
- HALO®: The most advanced of all lympho-hematopoietic assays and the basis for all other assays in the HALO® Family and all ATP bioluminescence-based assays developed by Preferred Cell Systems™. All HALO® assays incorporate fully standardized and validated readouts, yet can be learnt within a single day and are fast to use producing results within 15 minutes after assay standardization. All HALO® assays also incorporate proficiency testing and measurement assurance parameters.
- HALO® "Global" are research assays that allow 4-, 5- or 7-populations to be determined simultaneously, side-by-side.
2. Hematopoietic Cellular Therapy Applications
- STEMpredict™ is a 3-day predictive stem cell "quality" assay designed for cord blood, bone marrow and mobilized peripheral blood to differentiate high from low quality samples prior to cryopreservation. STEMpredict™ is the fastest stem cell assay available.
- HALO® QC is a 5 day predictive stem cell quality control assay to ensure the presence and yield of stem cells before and after and preparation procedure, e.g. fractionation, cryopreservation. HALO® QC has a Master File with the FDA.
- HALO® SC-IPS: A 7-day FDA compliant stem cell identity, purity and strength (potency) assay for all sources of hematopoietic cells for transplantation. HALO® SC-IPS has a Master File with the FDA.
- HALO® TE is a 5- day predictive Time to Engraftment assay to determine erythroid, neutrophil or platelet engraftment at any time after transplantation.
- HALO® PMT "Global" is a 4,- 5- or 7-population lympho-hematopoietic reconstitution assay.
3. In Vitro Hematotoxicity High-Throughput Screening and Testing
- HALO®-Tox HT: To determine the response of individual cell populations derived from different species and and tissues to new drug candidates or virtually any other compound, including xenobiotics, or perturbations. HALO®-Tox HT has been specifically designed to encompass routine high-throughput screening using 96- or 384-well plates.
- HALO®-Tox HT "Global" is the 4-, 5- or 7-population side-by-side version of the individual HALO®-Tox HT assays.
The development of the HALO® Family of assays led to the development of other ATP bioluminescence assays for many other tissues.