HALO® QC
A Standardized and Validated In Vitro Bioluminescence Quality Control Process Assay for Hematopoietic Stem Cell Therapy Products
Buy HALO® QC
Halo® QC
A Standardized and Validated Bioluminescence Assay to Monitor Lympho-Hematopoietic Reconstitution After Transplantation
| Description | Tissue | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Stem Cells | |||||
| SC-GEMM 1 | Any hematopoietic tissue | Low serum | K2-1QC-1 | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Any hematopoietic tissue | Low serum | K2-2QC-2 | 1 Kit | Stem Cells |
| SC-GEMM 1 | Any hematopoietic tissue | Serum-Free | K2SF-1QC-1 | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Any hematopoietic tissue | Serum-Free | K2SF-2QC-2 | 1 Kit |
Download the Quality Control Assay Flyer
HALO® QC - Uses, Benefits and Characteristics
- Ensure stem cell quality, number and yield before and after any key cellular therapy preparation, process or procedure (e.g. cell fractionation, cryopreservation etc.).
- For cord blood, bone marrow, mobilized peripheral blood or purified cells (e.g. CD34+, CD133+).
- Accurate and repeatable single dose, cell proliferation data provides stem cell quality control.
- Fully standardized, verified and validated according to FDA Guidelines.
- Proficiency testing is completed during assay standardization (see below). No additional, costly and time-consuming proficient testing required.
- Measurment assurance parameters provide proficiency and the trust in your results.
- Makes "quality" using TNC, viability, CD34 or CFU assays obsolete.
- Suspension Expansion Culture™ (SEC™) Technology provides high precision dispensing and makes the use of methylcellulose obsolete.
- 96-well plate format means smaller sample and reagent volumes with faster setup.
- Available to detect 1 or 2 primitive hematopoietic and/or lympho-hematopoietic stem cell populations.
- Short, 5-day incubation. Extend to 7 days for 2-3 fold greater sensitivity.
- Let the plate reader acquire and calculate results in 5 minutes or less.
- Compare stem cell preparation quality of cell therapy products over time and with other processing laboratories.
- Assay kits include everything needed to culture and measure stem cell quality. Just prepare and add cells.
- Time efficient and cost-effective.
- Easy to learn in just 1 day.
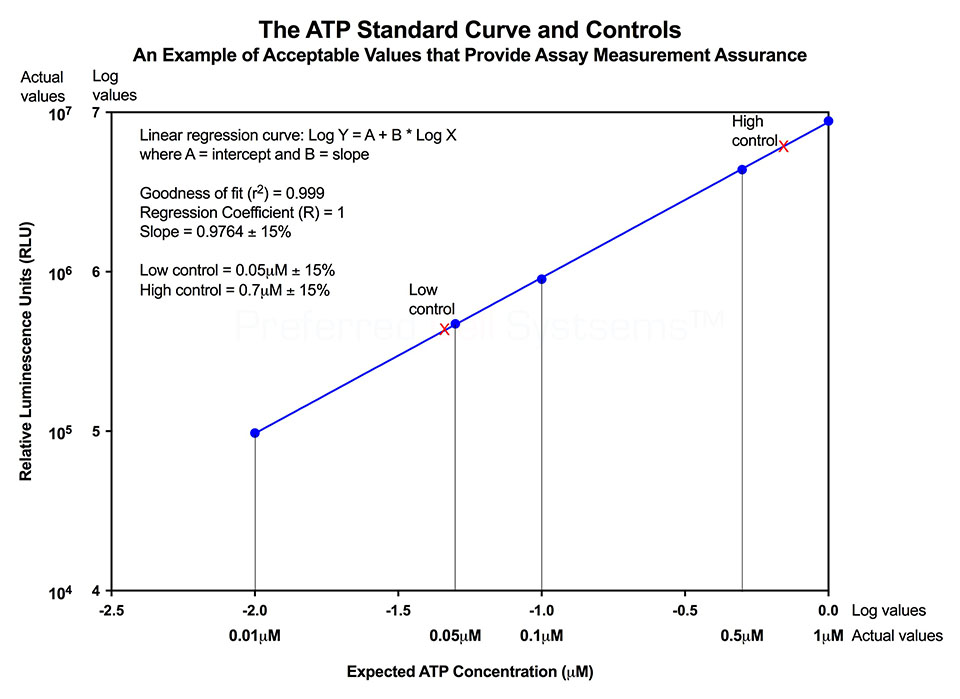
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- Mobilized peripheral blood
- Umbilical cord blood
- Bone marrow
- Purified cells from the above tissues
The recommended cell purity is a mononuclear cell (MNC) fraction or higher purity (see below). A total nucleated cell (TNC) fraction is not recommended as this contains high concentrations of cell impurities, such as red blood cells, neutrophils, platelets and other cells that dilute, mask and severely underestimate and even inhibit the detection of rare primitive stem cells.
- Standardization of the CFU-GM assay using hematopoietic growth factors. J. Hematotherapy:6:191-192 (1997)
- Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion. 48:620-628 (2008).
- Potency, Proliferation and Engraftment Potential of Stem Cell Therapeutics: The Relationship between Potency and Clinical Outcome for Hematopoietic Stem Cell Products. J Cell Sci Therapy. (2013).
- Detecting primitive hematopoietic stem cells in total nucleated and mononuclear cell fractions from umbilical cord blood segments and units. J Translational Medicine 13:94 (2015)
- Improving quality and potency testing for umbilical cord blood: A New Perspective. Stem Cells Translational Medicine. 4:967-973 (2015)
- Hematopoietic stem cell potency for cellular therapeutic transplantation. In: Hematopoietic Stem Cells, Ed. Rosana P. Camacho. Published by: InTech Open Access Publisher. ISBN 978-953-307-746-8. (2011).
- Measuring the Potency of a Stem Cell Therapeutic. In: Stem Cell Protocols. Methods in Molecular Biology, 1235, Ed. Rich IN. Published by Human Press (2015).
- Bioluminescence Potency Measurement of Cellular Therapy Products. In: Cellular Therapy: Principles, Methods, and Regulations, 2nd Edition, Eds. Areman EM and Loper K. Published by AABB.
- Present Cord Blood Testing Fails to Determine if the Stem Cells Used for Transplantation are of High Quality and PotencyThe Parents Guide to Cord Blood Foundation
- The Difference Between Stem Cell Viability and Potency: A Short Guide for Parent and Patients. The Parents Guide to Cord Blood Foundation
The cord blood and, more recently, the bone marrow stem cell transplantation community base all functional testing of their cellular therapy products on the total nucleated cell or TNC count. The total number of transplanted cells, the viability of those cells, the number of CD34+ cells in the fraction and the CFU colony count are all based on the TNC fraction. This fraction is produced by red blood cell reduction, but not depletion, and/or plasma reduction. The fraction still contains granulocytes and platelets and other cell impurities. The preparation of the TNC fraction is an instrument-based, closed and automated process into which a considerable amount of money has been invested by companies that have been told by the cord blood community that the TNC fraction is the only fraction needed. Indeed, so confident is the cord blood community that they are correct in using the TNC fraction, that they have taken no further interest in any other research that might prove this argument incorrect. Indeed, virtually all of the assumptions and conclusion drawn from decades of work using the TNC fraction, might actually be considered false.
The reason is simple. The cord blood and bone marrow transplantation community have based virtually all of their work on tests that are not only primitive, non-validated and obsolete, but more importantly, do not even detect, let alone quantitatively measure, the stem cells, without which, no stem cell transplantation procedure would be possible.
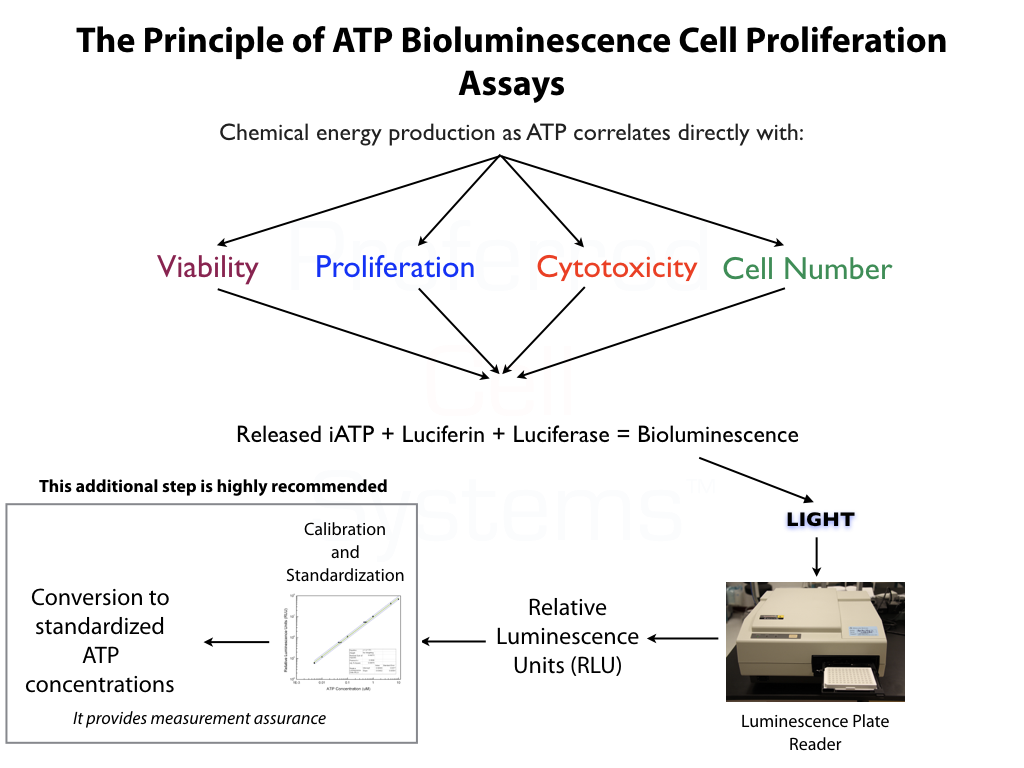
The diagram above shows how HALO® QC has been used to analyze the primitive hematopoietic stem cell content of 7 cord blood samples. Samples were obtained as the TNC fraction from different cord blood banks. An aliquot of each sample was removed and further fractionated to the mononuclear cell (MNC) fraction, thereby removing the majority of the RBCs, the granulocytes, platelets and other cell impurities. The cells from each fraction were then analyzed using HALO™ QC by quantitatively measuring the intracellular ATP, as a measure of cell viability and proliferation, after 5 days of incubation.
Three of the 7 seven samples analyzed, exhibited ATP values similar or below the acceptance limit of 0.04µM +- 15%. What does this mean? It means that a sample within this range might be able to sustain cell proliferation. Samples below this range are essentially metabolically dead. Samples above this range would viability and be capable of cell proliferation.
In the upper graph, 3 out of 7 TNC samples were within or below the acceptance level. If HALO® QC or any other quantitative assay from Preferred Cell Systems™ had been used to analyze these samples based on the TNC fraction, they would probably not have been considered for the Cord Blood Registry, since the CFU assay would have been negative. It would also have been highly likely that both the viability and CD34 content would have been below par, although the TNC count might have indicated otherwise. It should be noted that the cord blood community only collects cord blood units with a high TNC count and virtually regardless of any other indication. This assumption is based, but nowhere defined or published, that the higher the TNC count, the greater the possible number of stem cells, without actually measuring the stem cells. Indeed, neither the regulatory agencies (e.g. FDA) nor any of the standards organization, require assays to determine the presence and quantify the quality and potency of stem cells and therefore, no cord blood bank measures stem cells in the units they collect.
In contrast, all of the MNC fractions, exhibited ATP values greater than that of the acceptance range, albeit, to varying degrees, indicating not only the presence of primitive hematopoietic stem cells, but also their viability and capability of cell proliferation. What do these results indicate?
They indicate that using the TNC fraction masks and dilutes the all important stem cells present in the samples. It also indicates that basing the identity, purity and potency on the TNC fraction instead of the MNC fraction leads to false assumptions, incorrect interpretations and invalid conclusions, which, in turn, have led not only to considerable wastage of financial resources, but probably put patient's lives at risk.
The lower graph shows the direct correlation between TNC and MNC, a result to be expected since MNC are derived from TNC. However, this result again clearly demonstrates the advantage of using the MNC fraction and HALO® for all functional testing of cord blood, bone marrow and mobilized peripheral blood. This is why Preferred Cell Systems™ highly recommends using the MNC fraction for all testing purposes.
It might appear that using the TNC fraction is more rapid and cheaper in the short-term, but results in extremely serious consequences in the long-term, especially for patients.
Luminescence plate reader or multimode plate reader with "glow" luminescence measuring capability.
- HALO® QC Master Mix for SC-HPP and/or SC-GEMM and/or background control
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
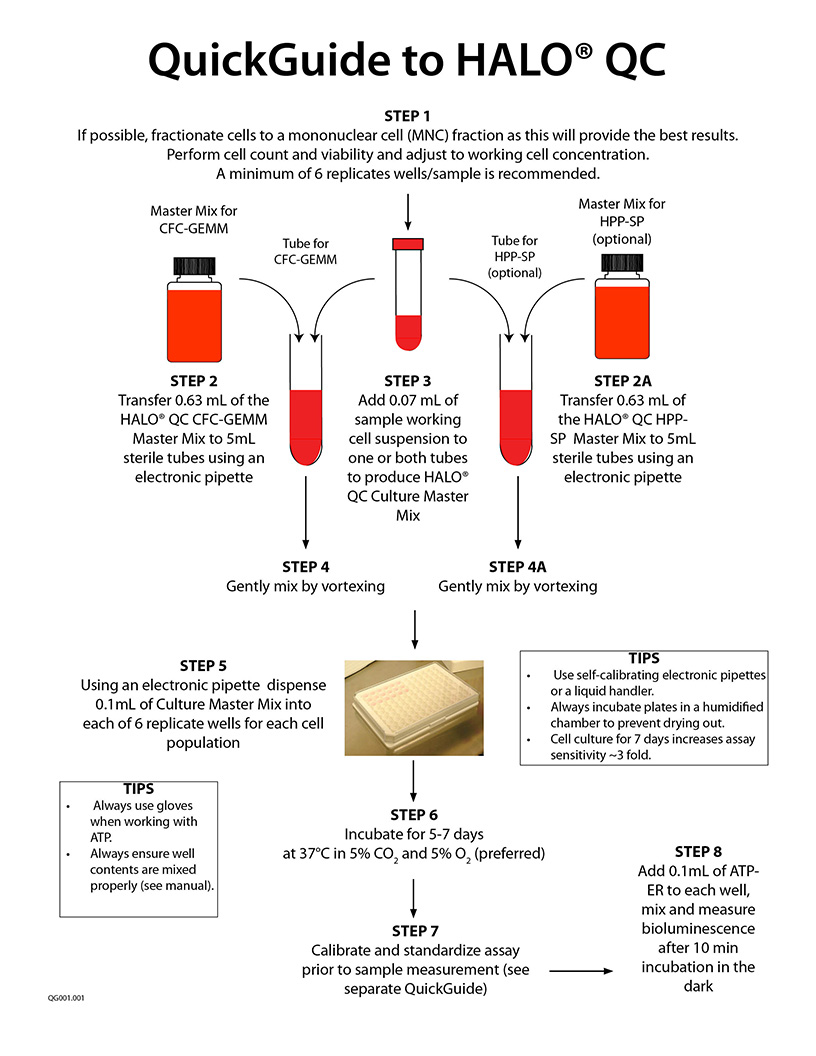
A specific video tutorial on using HALO® QC is not yet ready. Please scroll down and check out the QuickGuide and Technical Manual (if available). Below, are the links to perform the ATP bioluminescence readout:
How to Calibrate and Standardize an ATP Bioluminescence Assay - Part 1
How to Calibrate and Standardize an ATP Bioluminescence Assay - Part 2
Proficiency Testing for Hematopoietic Cellular Therapy Products
Download the ATP Optimization Kit Protocol for First-Time Users
Download the Luminometer Setup and RLU to ATP Conversion
Download SDS Sheet for HALO® QC Master Mix.
Download SDS Sheet for ATP Enumeration Reagent
Download SDS Sheet for ATP Standard and Controls Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM