HALO® RS & HALO® SC-IPS
A Standardized and Validated Bioluminescence
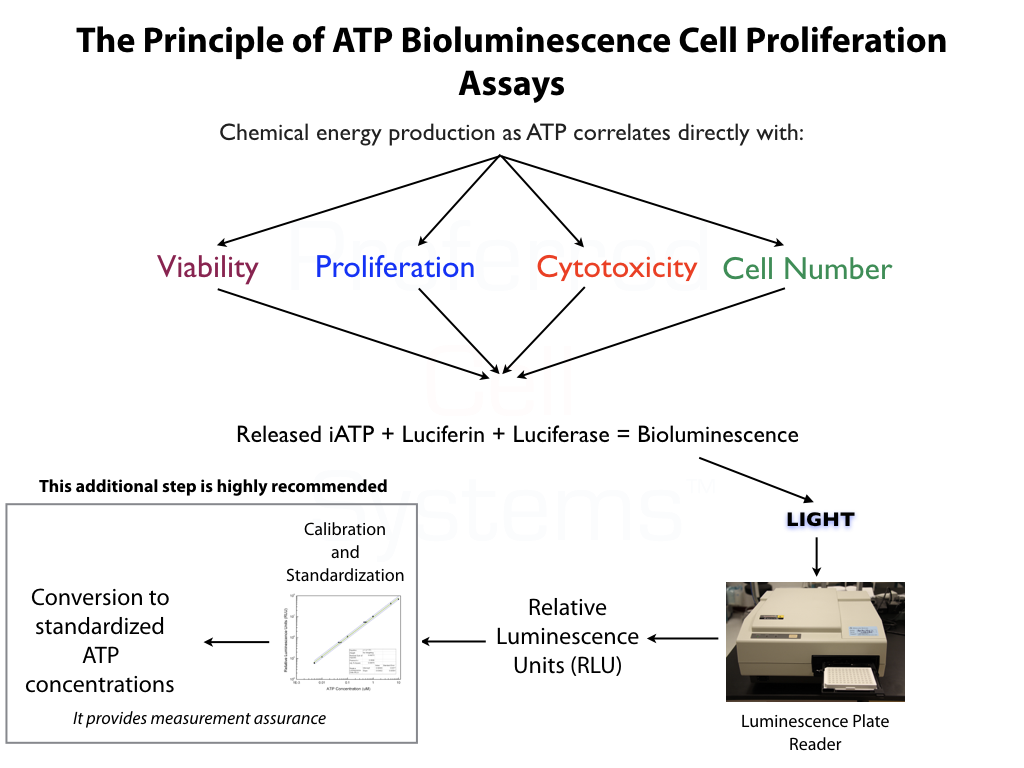
Predictive Engraftment Potential (Potency) Assay
Buy HALO® RS
Halo® RS
A Standardized and Validated Bioluminescence Assay to Establish Potency Reference Standards (RS) for Human Cells
| Description | Species Tissue | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Stem Cells | |||||
| SC-HPP 2 + SC-GEMM 1 | Bone marrow | Low serum | K2-PRS-1BM | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Bone Marrow CD34+ | Low serum | K2-PRS-1BM34 | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Cord blood | Low serum | K2-PRS-1CB | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Cord Blood CD34+ | Low serum | K2-PRS-1CB34 | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Mobilized peripheral blood | Low serum | K2-PRS-1mPB | 1 Kit | |
| SC-HPP 2 + SC-GEMM 1 | Mobilized peripheral blood CD34+ | Low serum | K2-PRS-1mPB34 | 1 Kit | Stem Cells |
| SC-HPP 2 + SC-GEMM 1 | Bone marrow | Serum-Free | K2SF-PRS-1BM | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Bone Marrow CD34+ | Serum-Free | K2SF-PRS-1BM34 | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Cord blood | Serum-Free | K2SF-PRS-1CB | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Cord Blood CD34+ | Serum-Free | K2SF-PRS-1CB34 | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Mobilized peripheral blood | Serum-Free | K2SF-PRS-1mPB | 1 Kit | SC-HPP 2 + SC-GEMM 1 | Mobilized peripheral blood CD34+ | Serum-Free | K2SF-PRS-1mPB34 | 1 Kit |
Halo® SC-IPS
A Standardized and Validated Bioluminescence Assay to Establish Potency Reference Standards (RS) for Human Cells
| Description | Species & Tissue | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Stem Cells | |||||
| SC-HPP 2 + SC-GEMM 1 | Any hematopoietic tissue | Low serum | K2-PQR-1 | 1 Kit | Stem Cells |
| SC-HPP 2 + SC-GEMM 1 | Any hematopoietic tissue | Serum-Free | K2SF-PQR-1 | 1 Kit | |
Download the Potency Assay Flyer
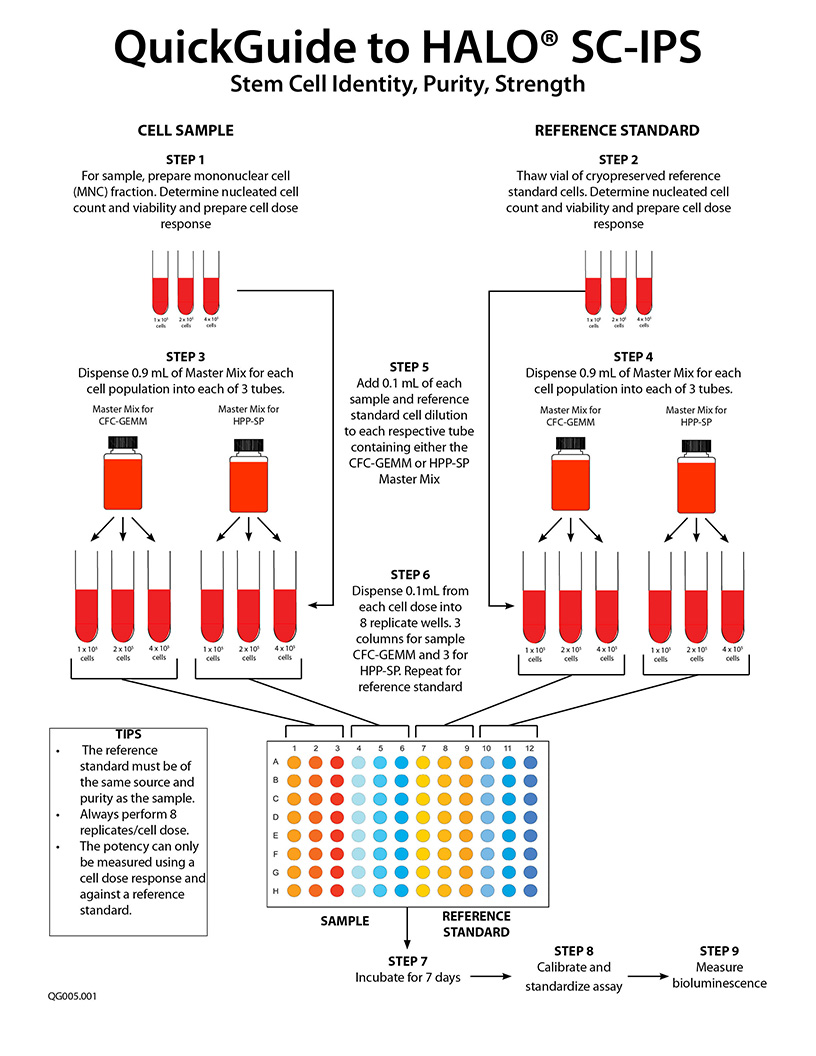
What Does SC-IPS Mean?
- SC = Stem Cell
- IPS = Identity, Purity, Strength. These are the parameters that the U.S. Food and Drug Administration (FDA) requires for traditional drugs and now biological drugs, such as umbilical cord blood and products for regenerative medicine.
- Identity defines the "active ingredients" that are responsible for the intended effect or response. For a hematopoietic cell therapy products, only the stem cells are the "active" ingredients and must therefore be identified.
- Purity defines whether the "active" ingredients are free of impurities and whether these impurities influence the intended effect or response. For hematopoietic cell therapy products, impurities can dilute and mask the identity of the "active" ingredients thereby leading to serious underestimation of the stem cell response.
- Strength is considered by the FDA to be equivalent to potency, which predicts the intended effect or response. The strength or potency of the "active" stem cell ingredients predicts stem cell engraftment prior to transplantation and therefore provide the measurement assurance to release the product for use in a patient.

- Accurate cell proliferation data provides stem cell potency (strength), identity, purity and quality information in a single assay.
- For cord blood, bone marrow, mobilized peripheral blood or purified cells (e.g. CD34+, CD133+ etc).
- Provide the transplantation physician with the most qualified information on the stem cell product in the shortest possible time prior to use.
- Establish in-house reference standards (using HALO® RS) that allow the potency ratio (measure of potency) to be determined.
- Fully compliant with FDA potency assay regulations and guidelines for hematopoietic cell therapy products.
- HALO® SC-IPS has a Master File with the FDA that can be referenced in an IND or BLA to the FDA. For more information, please contact Preferred Cell Systems™.
- Fully standardized, verified and validated according to FDA guidelines.
- Proficiency testing performed and completed during the assay standardization procedure. No additional costly and time-consuming proficiency testing required.
- Measurement assurance parameters provide proficiency and the trust in your results.
- Measurement of strength or potency is performed using a modified "Slope-Ratio Concentration-Response Model" standardized by the U.S. Pharmacopeia.
- Makes "potency" testing using TNC, viability, CD34 or CFU assays obsolete.
- Suspension Expansion Culture™ (SEC™) Technology provides high accuracy and precision and makes dispensing methylcellulose obsolete.
- 96-well plate format means smaller sample and reagent volumes with faster setup.
- Short 7-day incubation to predict engraftment potential.
- Let the plate reader acquire and calculate results in just 5 minutes or less.
- Directly compare stem cell potency, identity, purity and quality from different donors and processing procedures over time.
- Includes everything needed to culture and measure stem cell potency, identity, purity and quality. Just prepare and add cells.
- Time-efficient and highly cost-effective.
- Easy to learn in just 1 day.
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
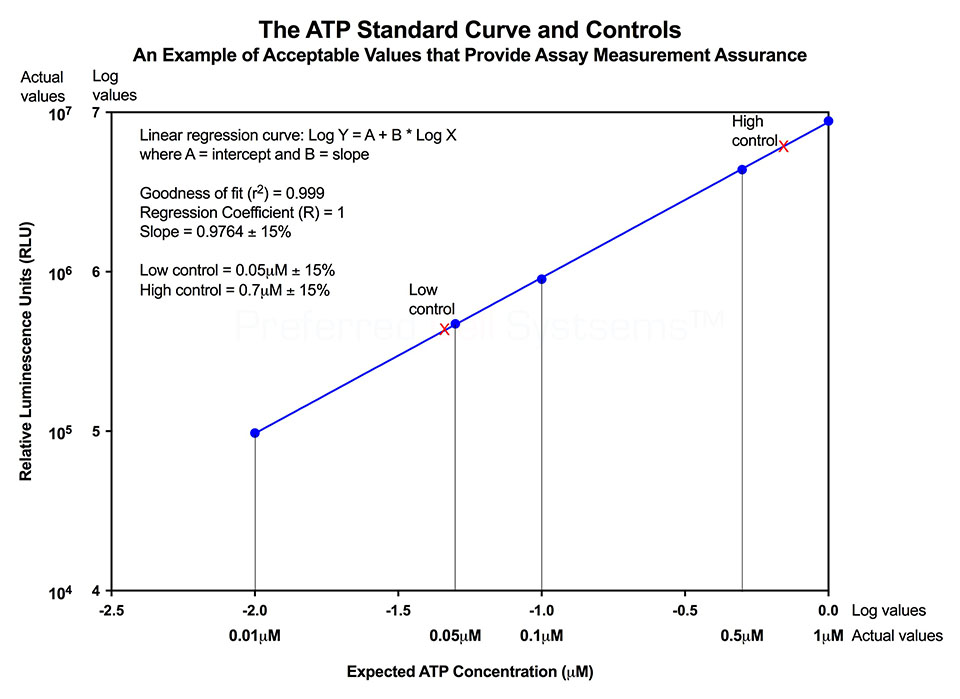
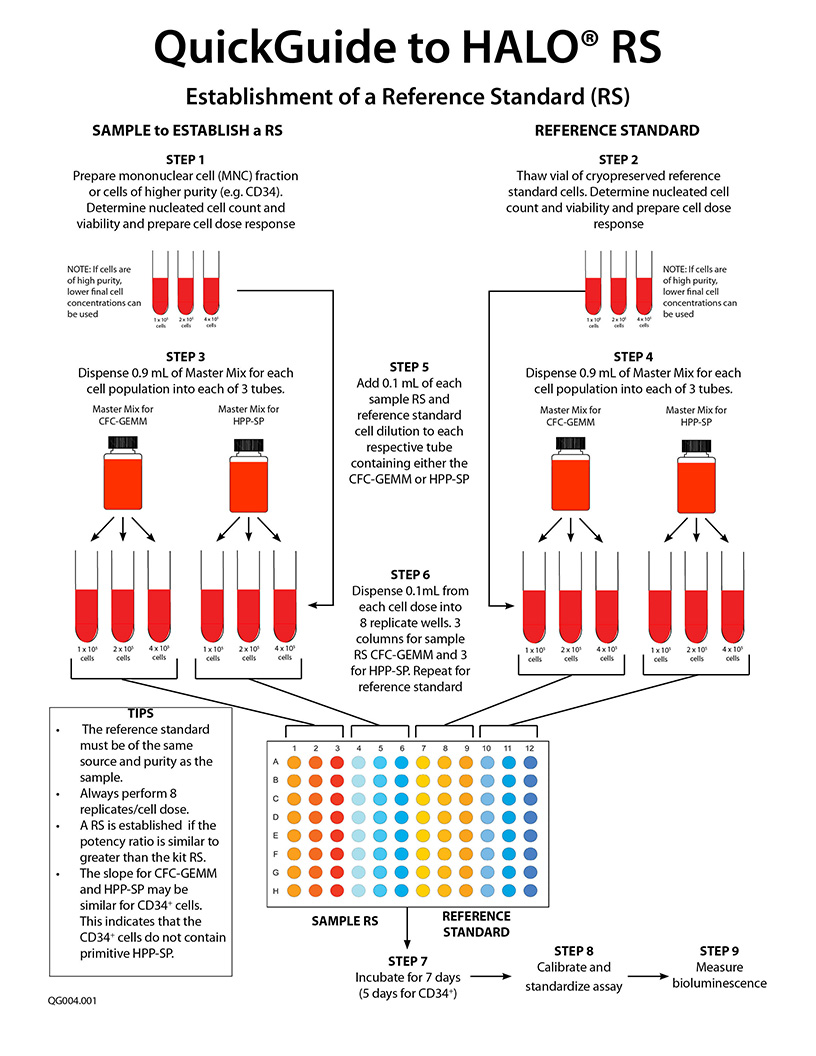
- To measure strength or potency, a reference standard (RS) of the same material as the sample is required so that the potency ratio can be determined. The inclusion of a RS to measure potency is an FDA requirement. Universal or global reference standards for cell therapy products are not available. As a result, it is necessary for individual centers to establish their own RS. To accomplish this, a standardized and validated assay platform is required.
- HALO® RS is used to establish your first in-house RS for a specific tissue. The assay includes a vial of cryopreserved cord blood, peripheral blood or bone marrow cells, at a specific purification, that are used to compare to your own preparation. HALO® RS is only required once to set up your first RS cell preparation. Preparation of all further reference standards can be establish using HALO® SC-IPS by comparing the biological activity of a newly prepared RS with a previous RS. (See below).
- To measure potency of hematopoietic cellular therapy product samples prior to use in a patient, HALO® SC-IPS is used. The in-house established RS is compared to that of the sample To do this, it is necessary to perform a cell dose response for both the sample and the RS for both stem cell populations. NOTE: A single cell dose tested for a single cell population does not constitute a potency assay.
- A minimum 3-point cell dose response for both the sample and the RS is performed for both stem cell populations for which culture reagents are provided in the HALO® SC-IPS assay kit. A dose response is required because the slope of the sample cell dose response compared to that of the RS provides the potency ratio. The potency of a reference standard is always 1.
- If the potency ratio of the sample is lower than 1, i.e. the RS, the cells being considered will probably not have sufficient potency to ensure engraftment. If the potency ratio is similar to or greater than 1, the sample exhibits a high potency and the probability is also high that engraftment will occur.
- NOTE: A total nucleated cell (TNC) preparation, a single cell dose, a single cell population that is not a stem cell population and lack of a RS does not constitute a potency assay. In addition, a non-validated assay that lacks measurement assurance parameters also does not consitute a potency assay.
- In vitro, hematopoietic multipotential stem cell (SC-GEMM 1)
- In vitro, lympho-hematopoietic primitive stem cell (SC-HPP 2)
- Mobilized peripheral blood
- Umbilical cord blood
- Bone marrow
- Purified cells from the above tissues
The recommended cell purity is a mononuclear cell (MNC) fraction or higher purity. A total nucleated cell (TNC) fraction is not recommended as this contains high concentrations of cell impurities, such as red blood cells, neutrophils, platelets and other cells that dilute, mask and severely underestimate, and even inhibit the detection of rare primitive stem cells.
- Standardization of the CFU-GM assay using hematopoietic growth factors. J. Hematotherapy:6:191-192 (1997)
- Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion. 48:620-628 (2008).
- Potency, Proliferation and Engraftment Potential of Stem Cell Therapeutics: The Relationship between Potency and Clinical Outcome for Hematopoietic Stem Cell Products. J Cell Sci Therapy. (2013).
- Detecting primitive hematopoietic stem cells in total nucleated and mononuclear cell fractions from umbilical cord blood segments and units. J Translational Medicine 13:94 (2015)
- Improving quality and potency testing for umbilical cord blood: A New Perspective. Stem Cells Translational Medicine. 4:967-973 (2015)
- Hematopoietic stem cell potency for cellular therapeutic transplantation. In: Hematopoietic Stem Cells, Ed. Rosana P. Camacho. Published by: InTech Open Access Publisher. ISBN 978-953-307-746-8. (2011).
- Measuring the Potency of a Stem Cell Therapeutic. In: Stem Cell Protocols. Methods in Molecular Biology, 1235, Ed. Rich IN. Published by Human Press (2015).
- Bioluminescence Potency Measurement of Cellular Therapy Products. In: Cellular Therapy: Principles, Methods, and Regulations, 2nd Edition, Eds. Areman EM and Loper K. Published by AABB.
- Present Cord Blood Testing Fails to Determine if the Stem Cells Used for Transplantation are of High Quality and PotencyThe Parents Guide to Cord Blood Foundation
- The Difference Between Stem Cell Viability and Potency: A Short Guide for Parent and Patients. The Parents Guide to Cord Blood Foundation
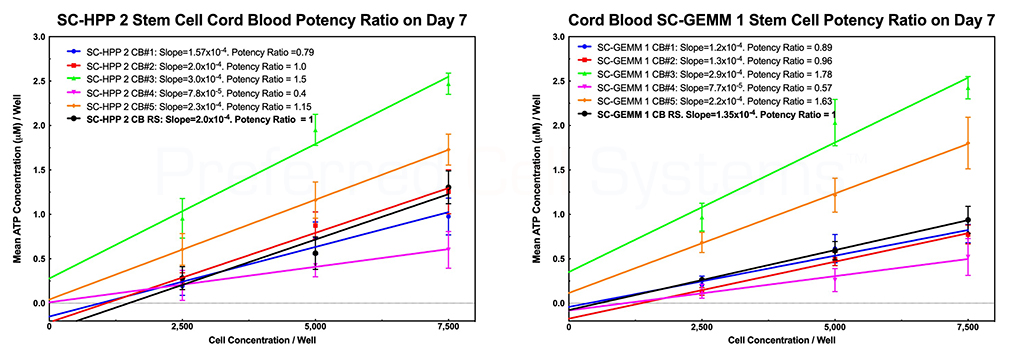
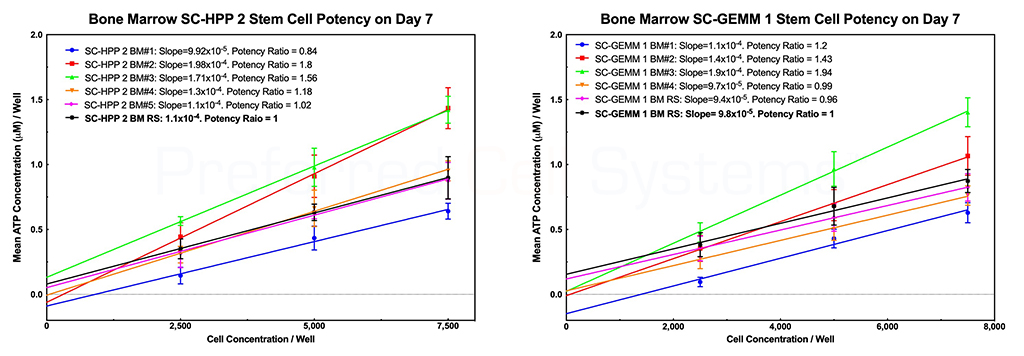
The graphs below demonstrate how umbilical cord blood and bone marrow stem cell potency ratios are determined. There are two primary requisites for measuring potency. The first is the requirement to perform a dose response of the sample. Potency cannot be measured using a single-point determination. The second is to compare the response of the sample with that of a reference standard. Since stem cells are the only cells that provide engraftment potential after transplantation, it is the potency or strength of the stem cells that needs to measured. All other cells are irrelevant. In addition, detecting just a single cell population will not provide the information required to determine engraftment potential. For this reason, the response of two stem cell populations are measured, namely the primitive lympho-hematopoietic stem cell, SC-HPP 2, and the primitive hematopoietic stem cell, SC-GEMM 1. Detecting downstream stem or progenitor cell populations will produce erroneous engraftment potential results. NOTE that potency is a predictive parameter. Potency predicts the dose. In this case, it is meant to predict the stem cell dose that will result in potential for stem cell engraftment. Measurement of potency cannot, therefore, be determined retrospectively.
The black dose response line in each graph represents the in-house umbilical cord blood reference standard. The slope of each cord blood sample dose response curve is compared to that of the reference standard to produce the potency ratio. The potency ratio is the measurement of potency. A sample with potency ratio lower than 1 (the potency of the reference standard) should not be used. A sample with a potency ration similar or greater than 1 can be used. Both stem cell populations should display a potency ration similar or greater than 1 to be used.
The name HALO® SC-IPS, indicates that the assay not only measures strength (S) or potency, but also identity and purity of the stem cells in the sample. The steepness of the stem cell dose response curve compared to that in other samples is a direct indication of the primitiveness and, therefore, the identity (I) of the stem cells in that sample. The steeper the curve, the more primitive the stem cells. In addition, if the purity (P) of the stem cells in a TNC, MNC or CD34+ sample were compared, the slope of the dose response curve would also determine the purity; the great the slope, the better the purity and, therefore, the more accurate the potency determination. Thus, the slope and therefore the purity of the cells would be: CD34+ > MNC > TNC. It is for this reason why the TNC fraction of cord blood should not and cannot be used to measure potency; it contains too many cellular impurities that mask the true potency of the sample.
- The simplest definition of potency is given by the European Medicines Agency (EMA) in accordance with the ICH Guideline 6QB as "the quantitative measure of biological activity based on the attribute of the product, which is linked to the relevent biological properties".
- This is in contrast to the definition of potency provided by the Food and Drug Administration (FDA) as "the specific ability or capacity of the product, as indicated by appropriate laboratory tests or by adequate controlled clinical data obtained through the administration of the product in the manner inteded, to effect a given result".
- The definition of potency by the FDA is applied primarily to traditional drugs and does not take into account the biology of cellular or gene therapeutics.
- Although "adequate controlled clinical data" producing a clinical outcome would be the ideal measurement of potency, the clinical outcome is usually the result of the product exhibiting adequate potency. This means that relying on a clinical outcome would be a retrospective result, which does not provide information on potency.
- Potency is a prospective or predictive measurement of the capacity of the cellular product to elicit a successful clinical outcome.
- For cellular and gene therapy products, measurng potency would predict engraftment potential.
- A Gold Standard potency assay would be one that provides direct clinical data, but in the majority of cases, this would be a retrospective result and does not provide the predictive information needed for a potency assay.
- Many consider a human-mouse model, e.g. a NOD-SCID mouse model to be a Gold Standard potency assay, but besides being extremely costly and lacking timely results, such models lack quantitative, standardized and validated potency information.
- For many reasons (see below), in vitro tests and/or assays that may have been used for decades in the cellular therapy arena are only considered potency assays by name alone, not by the information they provide.
- There is yet no Gold Standard potency assay for any cellular therapy or regenerative medicine product.
- None. Both the EMA and FDA use the terms "strength" and "potency" synonymously. Both terms apply to the therapeutic activity of the product.
- Potency is measured using one or more potency assays. These should be developed in parallel with the development of the cellular therapeutic.
- Potency is used to help characterize the product. As more biological characterization of the therapeutic is performed, the potency assay should incorporate these advances in knowledge.
- The earlier in development potency is measure using a specific potency assay, the better the ability to characterize the final product.
- Since potency is a prospective/predictive measurement, it must be performed between processing of the cells and prior to use in a patient.
- Many products used for cellular and gene therapy are cryopreserved.
- Although several studies have shown that cells can be kept cryopreserved for many years and still demonstrate functionality, assays used are insensitive, inaccurate and have questionabal reliability and reproducibility.
- Since the "active ingredients" (see below) are the key cellular entities that must be measured, it follows that damage to these entities during processing, cryopreservation and storage, prior to use is of utmost importance.
- Potency should, therefore, be determined, at an absolute minimum, prior to use in the patient.
- If possible, potency should also be determined shortly after cryopreservation of the cells to ensure that the "active ingredients" have not been damaged.
- NOTE: If the "active ingredients" are stem cells, there will be a 2-3 fold decrease in functionality between the fresh and cryopreserved cells.
- The "active ingredients" of a cellular or gene therapeutic are those cellular entities that result in the intended response, which, in many cases, is the successful clinical outcome.
- If the potency of the "active ingredients" are not measured, the outcome of the therapy cannot be predicted. Stated differently, if cells other than the "active ingredients" are measured, the assay is not measuring potency and outcome cannot be predicted.
- The "active ingredients" of any stem cell transplantation are the stem cells. No other cells are responsible for engraftment.
- According to the FDA, all "active ingredients" must be measured.
- However, for cellular or gene therapeutics, this is probably a physical impossibility (not to mention the cost involved), since the number of different "active ingredients" may not be known and, specific assays to measure all of the different "active ingredients" may not be available.
- However, measuring a single "active ingredient" may provide false positive or negative information. For example, if an assay can only measure the potency os a single active component, that component may not provide all of the necessary information to predict adequate potency. Thus, measuring the potency of mature stem cells only, will not provide the information to predict potential long-term reconstitution given by the potency of primitive stem cells.
- Therefore, the answer to this question is yes and no. Yes, because of regulatory requirements. No, because of the impracticality and cost. However, a minimum of 2 "active ingredients" should be measured.
- As stated above, measuring potency is a prospective or predictive indicator for a sucessful clinical outcome. Therefore, a potency assay is a release assay for the product (see below).
- A potency assay quantitatively measures the biological activity and capacity of the product.
- In order for the potency of a product to be acceptable, the potency assay must be validated according to specific regulatory guidelines. This means that the potency assay must not only include reference materials, standards and/or controls, but must document the accuracy, sensitivity, specificity, reliability and reproducibility.
- Since a potency assay is a validated assay, it provides the pre-define acceptable and/or rejection criteria for the product; i.e. it allow or rejects release of the product.
- A potency assay allows manufacturing conformity to be determined.
- A potency assay allows the stability of the product to be determined.
- A potency assay must indicate lack of adequate potency due to potential toxicity by the product itself or by impurities present in the product.
- According to the FDA, "no lot of any licensed product shall be released by the manufacturer prior to the completion of tests for conformity with standards applicable to such product, which include tests for potency, sterility, purity and identity. These requirement apply to all biological products, including autologous and single patient allogeneic products, where a lot may be defined as a single dose".
- To determine sterility, purity and identity, additional tests are usually required. However, in some cases (such as HALO®-SC-IPS), both identity and purity can be determined, together with potency, in the same assay.
- Identity is required to ensure that the "active ingredients" are actually measured.
- Purity is required to ensure that sufficient "active ingredients" are present in order to measure potency, since cellular impurities can otherwise result not only in a dampening of potency, but also a false interpretation of the potency.
- The purity of the final product is a determining factor in measuring potency.
- The more pure the final product, the better and more accurate the potency measurement.
- Many hematopoietic cellular therapy products are only fractionated to the total nucleated cell (TNC) fraction prior to cryopreservation. Although, upon thawing, many cell impurities are destroyed, e.g. granulocytes and platelets, those that are not destroyed and removed and other cell impurities result in a significant masking of the "active ingredients" with a corresponding false interpretation of the results.
- It has been scientifically demonstrated that further fractionation from the TNC to the mononuclear cell (MNC) fraction, signficantly improves the accuracy, sensitivity and selectivity of "active ingredient" potency.
- However, it should also be noted that further purification on MNCs to CD34+ cells, might improve the potency of mature stem cells, but the CD34+ fraction contains far few primitive lympho-hematopoietic stem cells.
- No. The potency of any material, regardless of whether it is a drug or cellular or gene therapeutics, can only be determined using a dose response.
- To measure potency of a sample, it should be compared to a reference standard of the same material and degree of purity. Otherwise, the measure of potency, the potency ratio, cannot be determined.
- The ratio of the sample to the reference standard provides the measurement of potency, the potency ratio.
- Remember, the potency defines the dose of the product.
- The potency of the reference standard (RS) or material is always 1.
- A potency ratio less than 1, indicates that more of the product would be required to produce the same response as the RS. In short, a sample with a potency ratio less than 1 would indicate a less than favorable response and probably should not be used in a patient.
- A potency ratio greater than 1 indicates that less of sample would be required to produce the same response as the RS. In other words, the probability of the product producing a favorable response would be high.
- A potency assay must be specific for the cell product and the "active ingredients" being measured. A potency assay should be developed according to regulatory guidelines.
- Several flow cytometric assays have been developed, but lack many of the requirements for a potency assay, e.g. lack of standards and controls, no reference standards, lack of a dose response relationship, lack of potency ratio determination. These are potency assays in name only.
- Other tests such as cell count, viability and cell-specific content number, e.g. CD34+ count, also suffer from the same lack of requirements as well as accuracy, specificity and reliability. These are far from potency assays.
- Viability, in particular, dye exclusion viability demonstrates a high degree of false positive results, especially where the "active ingredients" are stem cells. Viability is not a potency assay.
- Colony-forming unit assays (CFU) have been considered a potency assay for several years, despite lack of standardization, validation and measurement assurance parameters. In addition, low accuracy, specificity, reliability and reproducibility all indicate that the CFU assay should not and can not be used as a potency assay.
- At the present time, no. Although in vitro expanded cells are being used in clinical trials etc. unless a specific assay has been developed for the "active ingredients" comprising the expanded cells, there is no definitive potency assay available.
- For example, CD34+ cells from cord blood or normal or mobilized peripheral blood might be starting point for an in vitro cell expansion procedure. However, at the end of culture, few, if any, CD34+ cells, let alone stem cells, will be present. If the response capacity of the expanded cells cannot be measured, it is unlikely that the potency can be quantified unless it can be compared to a known response.
Luminescence or multimode plate reader capable of measuring "glow" luminescence.
HALO®-RS Kit Contents:
-
Vial of cryopreserved reference standard umbilical cord blood, mobilized peripheral blood, bone marrow mononuclear cells (MNC) or purified cells (CD34 ) from the respective tissue
- HALO® Master Mixes for both SC-HPP 2 and SC-GEMM 1 stem cell populations
- ATP standards
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plate(s)
- Non-sterile, 96-well plate(s)
- Sterile, adhesive foil covers
HALO® SC-IPS Kit Contents
- HALO® SC-IPS Master Mixes for SC-HPP 2 and SC-GEMM 1 stem cell populations
- ATP standard
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
To understand and see how a potency assay is performed, please view the following Instructional Videos (Tutorials):
How To Measure Identity, Purity and Strength of Cellular Therapeutic Products Using HALO® SC-IPS.
How to Calibrate, Standardize and Process Samples for Any ATP Bioluminescence Assay (Part 1)
How to Calibrate, Standardize and Process Samples for Any ATP Bioluminescence Assay (Part 2)
Proficiency Testing for Hematopoietic Cellular Therapy Products
Download the ATP Optimization Kit Protocol for First-Time Users
Download the Luminometer Setup and Conversion of RLU to ATP Protocol Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM