HemoLIGHT™ PCA
The Absorbance Progenitor Cell Assay (PCA)
that Replaces Methylcellulose CFU for the
Cell Processing Laboratory
Buy HemoLIGHT™ PCA
HemoLIGHT™ PCA
An Easy and Rapid Methylcellulose-Free Absorbance Progenitor Cell Assay for the Cell Processing Laboratory
| Cell Population | MethoCult Equivalent | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Stem Cells | |||||
| SC-GEMM 3 | H4435 "Enriched" | Low serum | K4-PCA5-1 | 1 Kit | |
| SC-GEMM | Methocult "Express" | Low serum | K4-PCA6-1 | 1 Kit | |
| SC-GEM 3 | H4434 "Classic" | Low serum | K4-PCA1-1 | 1 Kit | |
| SC-GEM 2 | H4034 "Optimal" | Low serum | K4-PCA2-1 | 1 Kit | |
| Progenitor Cells | |||||
| P-GM 1 | H4534 "Classic" | Low serum | K4-PCA3-1 | 1 Kit | |
| P-GM 2 | H4035 "Optimal" | Low serum | K4-PCA4-1 | 1 Kit | Stem Cells |
| SC-GEMM 3 | H4435 "Enriched" | Serum-Free | K4SF-PCA5-1 | 1 Kit | SC-GEMM | Methocult "Express" | Serum-Free | K4SF-PCA6-1 | 1 Kit | SC-GEM 3 | H4434 "Classic" | Serum-Free | K4SF-PCA1-1 | 1 Kit | SC-GEM 2 | H4034 "Optimal" | Serum-Free | K4SF-PCA2-1 | 1 Kit |
| Progenitor Cells | |||||
| P-GM 1 | H4534 "Classic" | Serum-Free | K4SF-PCA3-1 | 1 Kit | |
| P-GM 2 | H4035 "Optimal" | Serum-Free | K4SF-PCA4-1 | 1 Kit | |
Download the Progenitor Cell Assay Flyer
How to Use HemoLIGHT™ PCS Video Tutorial
To learn how to setup and use HemoLIGHT™ PCA, click on the video links below:
How to Perform a Progenitor Cell Assay (PCA) - An Alternative to All Methylcellulose CFU Assays

- Determine the number of viable cells in proliferation.
- An alternative to, or replacement for the classic colony-forming unit (CFU) assay.
- FDA, FACT and AABB alternative assays.
- Replaces the CFU assay with an instrument-based, quantitative assay.
- No colony counting required.
- Uses an absorbance ELISA plate reader available in most laboratories.
- Incorporates the same growth factor cocktails used in MethoCult® reagents for direct assay-to-assay comparisons and correlation.
- Non-subjective, instrument-based and quantitative assay system.
- Results in 7 days depending.
- Faster and easier to use than any traditional CFU assay, even using automated colony counting.
- Simple, time efficient, flexible and more cost effective than methylcellulose CFU assays.
- Complete assay kit; includes everything needed to culture and obtain results.
- Incorporates proven Suspension Expansion Culture™ (SEC™) Technology.
| HemoLIGHT™ PCA Number | HemoLIGHT™ PCA Cell Population |
Equivalent CFU Cell Population | Equivalent MethoCult™ Reagent |
Growth Factor Cocktail |
|---|---|---|---|---|
| PCA1 | SC-GEM 3 | CFC-GEM 3 | H4434 "Classic" | EPO, GM-CSF, IL-3, SCF |
| PCA2 | SC-GEM 2 | CFC-GEM 2 | H4034 "Optimum" | EPO, GM-CSF, G-CSF, IL-3, SCF |
| PCA3 | P-GM 1 | GM-CFC 1 | H4534 "Classic" | GM-CSF, IL-3, SCF |
| PCA4 | P-GM 2 | GM-CFC 2 | H4035 "Optimum" | GM-CSF, G-CSF, IL-3, SCF |
| PCA5 | SC-GEMM 3 | CFC-GEMM 3 | H4435 "Enriched" | EPO, GM-CSF, G-CSF, IL-3, IL-6, SCF TPO(#) |
| PCA6 | SC-GEMM | CFC-GEMM | Methocult "Express" | Recombinant cytokines EPO |
(#) denotes that the Preferred Cell Systems™ formulation of this product includes TPO that stimulates the production of megakaryopoiesis. MethoCult™ and other competitor formulations of this specific product do not include TPO, do not stimulate the production of megakaryocytes and therefore do not detect the CFC-GEMM stem cell population.
*denotes that assay kits are available with 1, 2 or 4, 96-well plates
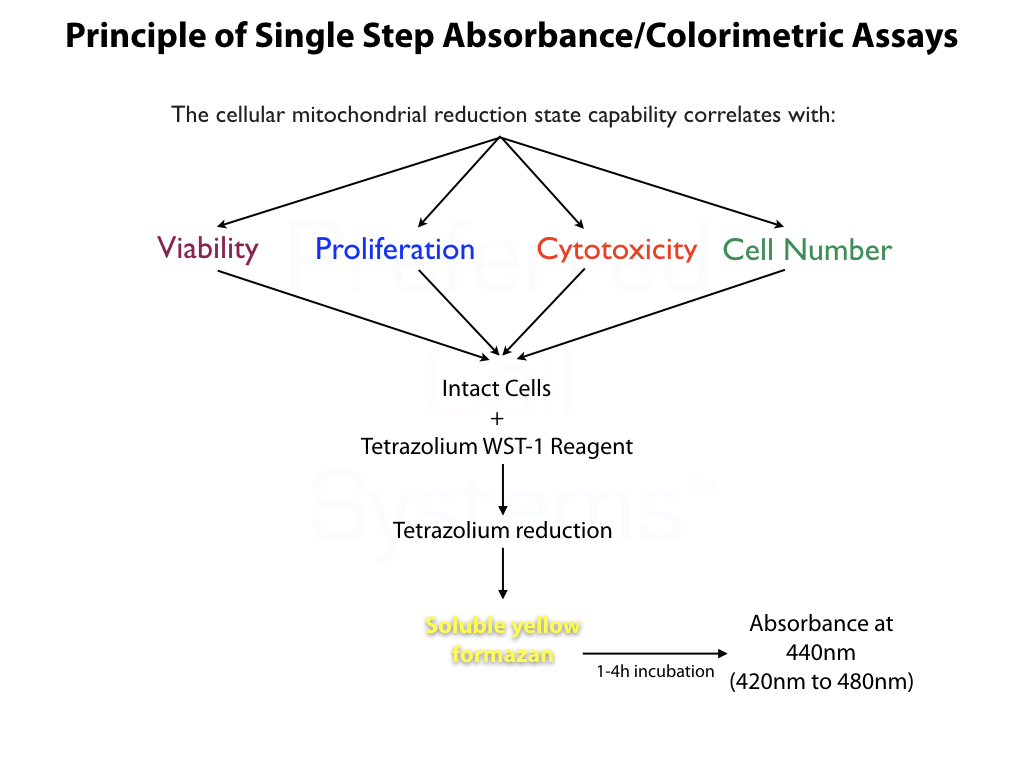
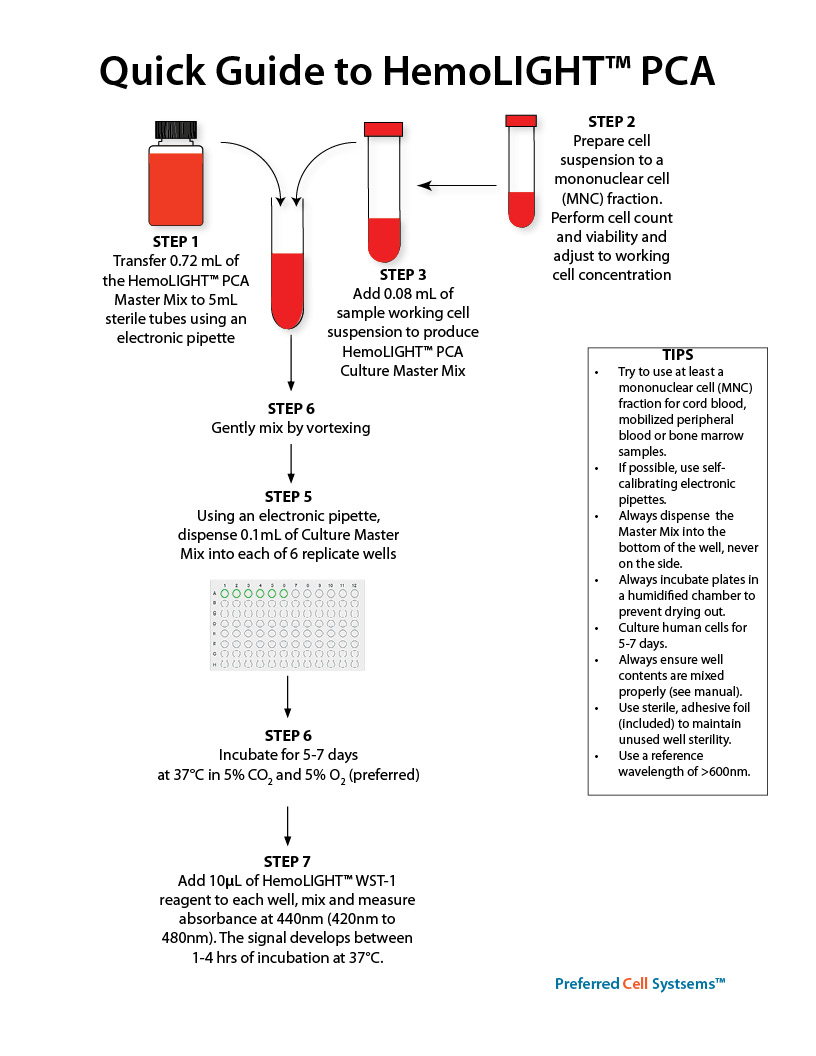
- Incorporates a terazolium WST-1 reagent that is reduced to a soluble yellow formazan, which can be measured at an maximum absorbance of 440nm (420nm to 480nm) in an absorbance plate reader.
- After culture, add 10μl of the WST-1 reagent, mix and read absorbance at 440nm after 1-4 hours in a 96-well plate reader. Plates can be returned to incubator and re-read at a later time for increased sensitivity.
- No solubilization step as in a MTT reaction. Replaces MTT assays.
- Mobilized peripheral blood
- Umbilical cord blood
- Bone marrow
- Purified cell populations for any of the above tissues
- Standardization of the CFU-GM assay using hematopoietic growth factors. J. Hematotherapy:6:191-192 (1997)
- Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion. 48:620-628 (2008).
- Potency, Proliferation and Engraftment Potential of Stem Cell Therapeutics: The Relationship between Potency and Clinical Outcome for Hematopoietic Stem Cell Products. J Cell Sci Therapy. (2013).
- Detecting primitive hematopoietic stem cells in total nucleated and mononuclear cell fractions from umbilical cord blood segments and units. J Translational Medicine 13:94 (2015)
- Improving quality and potency testing for umbilical cord blood: A New Perspective. Stem Cells Translational Medicine. 4:967-973 (2015)
- Hematopoietic stem cell potency for cellular therapeutic transplantation. In: Hematopoietic Stem Cells, Ed. Rosana P. Camacho. Published by: InTech Open Access Publisher. ISBN 978-953-307-746-8. (2011).
- Measuring the Potency of a Stem Cell Therapeutic. In: Stem Cell Protocols. Methods in Molecular Biology, 1235, Ed. Rich IN. Published by Human Press (2015).
- Bioluminescence Potency Measurement of Cellular Therapy Products. In: Cellular Therapy: Principles, Methods, and Regulations, 2nd Edition, Eds. Areman EM and Loper K. Published by AABB.
- Present Cord Blood Testing Fails to Determine if the Stem Cells Used for Transplantation are of High Quality and PotencyThe Parents Guide to Cord Blood Foundation
- The Difference Between Stem Cell Viability and Potency: A Short Guide for Parent and Patients. The Parents Guide to Cord Blood Foundation
- HemoLIGHT™ PCA Master Mix
- WST-1 reagent
- Sterile, clear, 96-well plate(s)
- Sterile, adhesive cover foils to maintain sterility of unused wells