The Keystone Assay™
by
Preferred Cell Systems™
A 1-Day Functional Quality Assay for
Hematopoietic Cellular Therapy Products
Hematopoietic Cellular Therapy Products
Buy The Keystone Assay
The Keystone Assay
A 1-day hematopoietic functional quality assay for cord blood and bone marrow
| Cell Population | Species | Catalog Number | ||
|---|---|---|---|---|
| The Keystone Assay | Keystone Cell Population | Human cord blood, mobilized peripheral blood, bone marrow | K2-KSA-1 |
Where Does the Term "Keystone" Come From?
The term "Keystone" was coined by Robert Paine in 1966. He observed that the ecosystem declined in intertidal pools as the number of predators in those pools decreased. His research led to the establishment of keystone species in the ecosystem that exert a down influence to prevent lower species from monopolizing critical resources. The keystone theory describing the ecological relationship between species has been supported by numerous publications since Paine's seminal publication in 1969.
What is The Keystone Cell?
The Keystone can be one or more cells that, if removed or damaged, can affect the response and functioning of a whole biological system, in this case, the hematopoietic system.
What is The Keystone Assay™?
The Keystone Assay™ quantitatively measures the response of a single Keystone Cell Population™ and allows the researcher to distinguish between high and low quality cellular therapy product samples within 24 hours.
Why Use The Keystone Assay™?For years AABB, ISCT and the Cord Blood Association have called for a fast functional assay to measure the quality and potency of bone marrow and cord blood samples prior to cryopreservation and when a donor product has been identified for transplantation. The call for such an assay has never been taken up by the cellular therapy community because they have always maintained that nucleated cell count, viability, CD34 content and the CFU assay (this being the only functional assay, but taking 14 days to complete) are sufficient. Thus, despite the call for "cutting-edge research", the banner has never been accepted.
Only Preferred Cell Systems™ has, once again, shown how "cutting-edge research" and "innovative science" can push the boundaries of cellular therapy testing.
Based on previous proven technology, Preferred Cell Systems™ spent more than 2 years developing the first, ever, 1-day hematopoietic in vitro quality functional assay for cord blood and bone marrow samples.
These samples can now easily be analyzed within 24 hrs to demonstrate whether the sample has high or low quality and, thereby, provide quantitative and reliable data to indicate that if the product is cryopreserved, it can be thawed and will demonstrate sufficient functional quality that will allow its use in a patient.
What are the Benefits of the Keystone Assay™?
Only Preferred Cell Systems™ has, once again, shown how "cutting-edge research" and "innovative science" can push the boundaries of cellular therapy testing.
Based on previous proven technology, Preferred Cell Systems™ spent more than 2 years developing the first, ever, 1-day hematopoietic in vitro quality functional assay for cord blood and bone marrow samples.
These samples can now easily be analyzed within 24 hrs to demonstrate whether the sample has high or low quality and, thereby, provide quantitative and reliable data to indicate that if the product is cryopreserved, it can be thawed and will demonstrate sufficient functional quality that will allow its use in a patient.
- A simple, easy-to-use, 1-day, quantitative functional assay.
- The assay kit comes complete with everything needed to perform the assay. Just prepare and add cells.
- No colony counting and far less expensive instrumentation, allowing anyone in the laboratory to perform the assay.
- Requires only a very small sample of fractionated cells (see below).
- Can be used for cord blood, bone marrow and, almost certainly, peripheral blood.
- Incorporates the most sensitive readout technology, presently available, to measure Keystone cell proliferation.
- Exceptionally high precision, reliability and reproducibility.
- Fully standardized and validated.
- Incorporates proficiency testing technology and measurement assurance parameters to indicate that the assay is functioning correct and that the results are trustworthy.
- Can be used in high-throughput mode for large numbers of samples using either 96- or 384-well plates.
In its simplest form, a single dose of a mononuclear cells suspension from human cord blood, bone marrow or peripheral blood (normal or mobilized) is cultured for 24 hours (and can be extended to 3 days, if necessary) in the Keystone Reagent in wells of a 96-well plate. To measure the response of this rare, primitive cell type and compare it to the response of a control cell suspension, a calibrated and standardized ATP bioluminescence signal detection system is used together with a luminescence plate reader. This detection system provides the greatest sensivity, accuracy, reliability and reproducibility that is needed to detect proliferation of these rare cells.
How Many Cells are Needed to Perform The Keystone Assay™?
This depends on the number of replicate wells prepared. Since a rare population is being measured, it is suggested to perform a minimum of 6 replicates wells/sample, although 8 replicates/sample would be statistically better. Each well will contain 5,000 MNCs in a total volume of 0.1mL. Therefore, for 6 replicates, a total of 300,000 cells/mL would be required.
What is The Keystone Reagent?
The Keystone Reagent contains a proprietary cocktail of growth factors that has been chosen out of more than 15 different growth factor cocktails to reliably and reproducibly stimulate The Keystone Cell Population™. The Keystone Reagent and a Control Reagent are included with The Keystone Assay Kit.
What are the Contents of The Keystone Assay™ Kit?
The Keystone Assay™ Kit contains everything you need to perform the assay. No cells are included with the assay kit.
What Instrument is Needed for The Keystone Assay™?
- The Keystone Reagent
- The Keystone Control Reagent
- Base medium (to dilute the ATP standard)
- ATP-Enumeration Reagent (ATP-ER)
- ATP Standard
- ATP Controls
- Sterile 96-well plate
- Non-sterile 96-well plate (used for calibration and standardization)
- Sterile adhesive foils
- Instruction manual
As mentioned above, to perform The Keystone Assay™, a luminescence plate reader is required. This should measure the "glow" bioluminescence signal that is produced. Bioluminescence is used because it is the most sensitive signal detection system to measure the rare Keystone Cell Population. In addition, in contrast to fluorescence or absorbance, the bioluminescence signal can be calibrated and standardized to re-validate the assay so that it complies with regulatory guidelines.
What is the Throughput of The Keystone Assay™?
The Keystone Assay™ employs an 96-well plate format allowing normal pipettes, multichannel dispensers and even liquid handler (robots) to be used. This allows fast, error-free setup and processing.
If large numbers of samples are analyzed, the assay is available, upon request, with 384-well plates. In this case, a liquid handler is a requirement, but this can be used for all reagent dispensing.
Keystone Assay™ Sample Data?
If large numbers of samples are analyzed, the assay is available, upon request, with 384-well plates. In this case, a liquid handler is a requirement, but this can be used for all reagent dispensing.
The data below show the time course for detecting The Keystone Cell Population™ from bone marrow and umbilical cord blood MNCs. They also show how The Keystone Assay™ can functionally differentiate, on day 1 of culture, high from low quality bone marrow or cord blood samples.
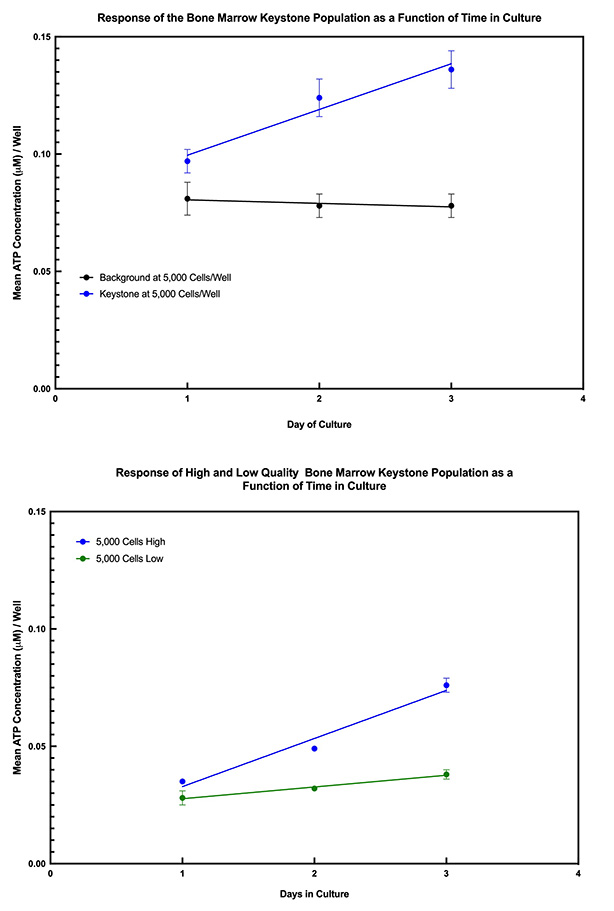
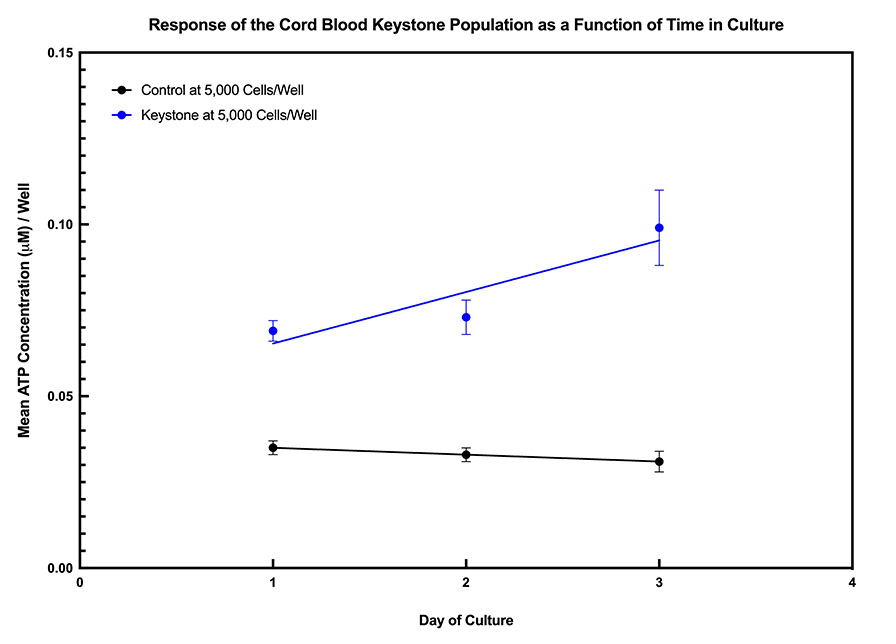
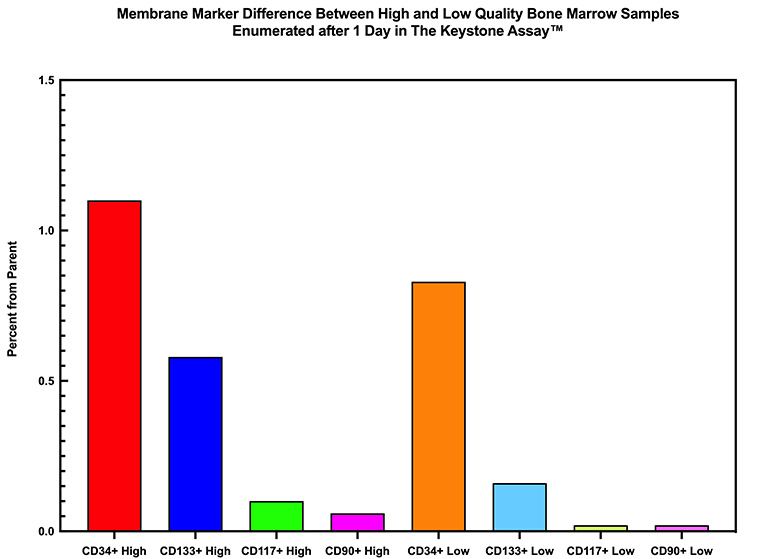
The difference between high and low quality bone marrow samples can also be seen based on single membrane marker detection when The Keystone Cell Population™ is analyzed by flow cytometry after 1 day in culture.
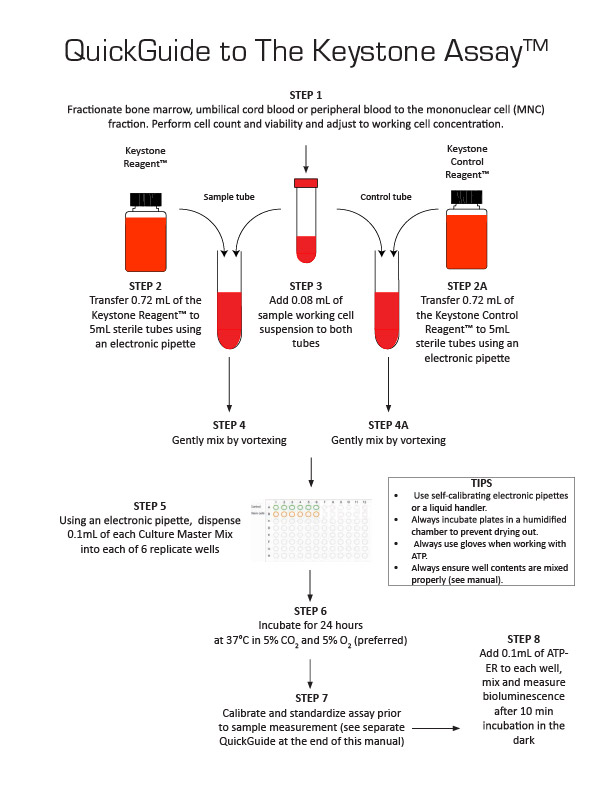
QuickGuide to The Keystone Assay™


The difference between high and low quality bone marrow samples can also be seen based on single membrane marker detection when The Keystone Cell Population™ is analyzed by flow cytometry after 1 day in culture.