HemoGLO™ PCA
The Bioluminescence Progenitor Cell Assay (PCA)
that Replaces Methylcellulose CFU for the
Cell Processing Laboratory
| Description | Catalog Number | Quantity |
|---|---|---|
| An Add-On Kit for Any Bioluminescence "GLO" Assay | K-ATPSC-1 | 1 Kit |
HemoGLO™ PCA
An Easy and Rapid Methylcellulose-Free Bioluminescence Progenitor Cell Assay for the Cell Processing Laboratory
| Cell Population | MethoCult Equivalent | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Stem Cells | |||||
| SC-GEMM 3 | H4435 "Enriched" | Low serum | K6-PCA5-1 | 1 Kit | |
| SC-GEMM | MethoCult "Express" | Low serum | K6-PCA6-1 | 1 Kit | |
| SC-GEM 3 | H4434 "Classic" | Low serum | K6-PCA1-1 | 1 Kit | |
| SC-GEM 2 | H4034 "Optimal" | Low serum | K6-PCA2-1 | 1 Kit | |
| Progenitor Cells | |||||
| P-GM 1 | H4534 "Classic" | Low serum | K6-PCA3-1 | 1 Kit | |
| P-GM 2 | H4035 "Optimal" | Low serum | K6-PCA4-1 | 1 Kit | Stem Cells |
| SC-GEMM 3 | H4435 "Enriched" | Serum-Free | K6SF-PCA5-1 | 1 Kit | SC-GEMM | Metocult "Express" | Serum-Free | K6SF-PCA6-1 | 1 Kit | SC-GEM 3 | H4434 "Classic" | Serum-Free | K6SF-PCA1-1 | 1 Kit | SC-GEM 2 | H4034 "Optimal" | Serum-Free | K6SF-PCA2-1 | 1 Kit |
| Progenitor Cells | |||||
| P-GM 1 | H4534 "Classic" | Serum-Free | K6SF-PCA3-1 | 1 Kit | |
| P-GM 2 | H4035 "Optimal" | Serum-Free | K6SF-PCA4-1 | 1 Kit | |

Download the HemoGLO™ PCA Flyer

- HemoGLO™ PCA replaces all obsolete methylcellulose colony-forming unit (CFU) assays with easy and rapid setup and quantitative enumeration.
- Let the plate reader acquire and calculate results in 5 minutes or less for a 96-well plate. NO COLONY COUNTING NECESSARY.
- If you want to know the differentiation status, combine HemoGLO™ PCAwith flow cytometry.
- 5-7 day cell culture incubation. Half the time or less than a CFU assay.
- NO METHYLCELLULOSE. Suspension Expansion Culture™ (SEC™) Technology makes methylcellulose obsolete.
- SEC™ Technology allows normal pipettes/dispensers to be used. NO INACCURATE SYRINGE DISPENSING.
- SEC™ Technology and the ATP bioluminescence provides the most sensitive, accurate and, above all, reliable and reproducible results than any CFU assay can offer.
- Use with cord blood, bone marrow, mobilized peripheral blood or purified cells (e.g. CD34+ or CD133+).
- Direct correlation with CFU. (See below. Correlation of HALO® PCA with the CFU assay).
- Similar growth factor cocktails to MethoCult™ and other methylcellulose reagents, but far superior cell growth.
- 96-well plates included in the kit. Smaller sample and reagent volume. Faster setup.
- Detect primitive stem and progenitor cells easily and efficiently.
- Assay kits include everything you need to culture and measure cell viability, proliferation and growth. Just prepare and add cells.
- HemoGLO™ PCA saves time and labor allowing personnel to perform other important activities.
- Learn HemoGLO™ PCA in just a few hours.
- HemoGLO™ PCA and its sister assays, HemoFLUOR™ PCA and HemoLIGHT™ PCA, are priced similar to those of MethoCult™ and other methylcellulose assays.
- If you need to standardize and/or validate the assay, perform proficiency testing or need measurement assurance parameters, use the HemoGLO™ PCA Assay Standardization Kit (Cat. No. K6-ASK-1). If you plan to standardize HemoGLO™ PCA routinely, please consider purchasing the equivalent HALO® Assay Kit.
However, despite numerous attempts to try and mitigate the flaws inherent in the CFU assay as well as to try and standardize the assay, there is also a legacy of failure. The introduction of dubious standards and costly and non-informative proficiency testing have also failed to improve the quality of results obtained from the CFU assay.
Preferred Cell Systems™ has attacked these failures head-on to first produce the HALO® Family of Assays and more recently HemoGLO™ PCA, HemoFLUOR™ PCA and HemoLIGHT™ PCA.
HemoGLO™ PCA, is a more simple version of HALO® that still incorporates Suspension Expansion Culture™ (SEC™) Technology and the most sensitive and accurate signal detection readout available, namely ATP bioluminescence. HemoGLO™ PCA joins its sister assays, HemoFLUOR™ and HemoLIGHT™ in providing sensitive, accurate and, above all, reliable and reproducible results for the cell processing laboratory at a similar or equal price compared to MethoCult™ or other methylcellulose reagents.
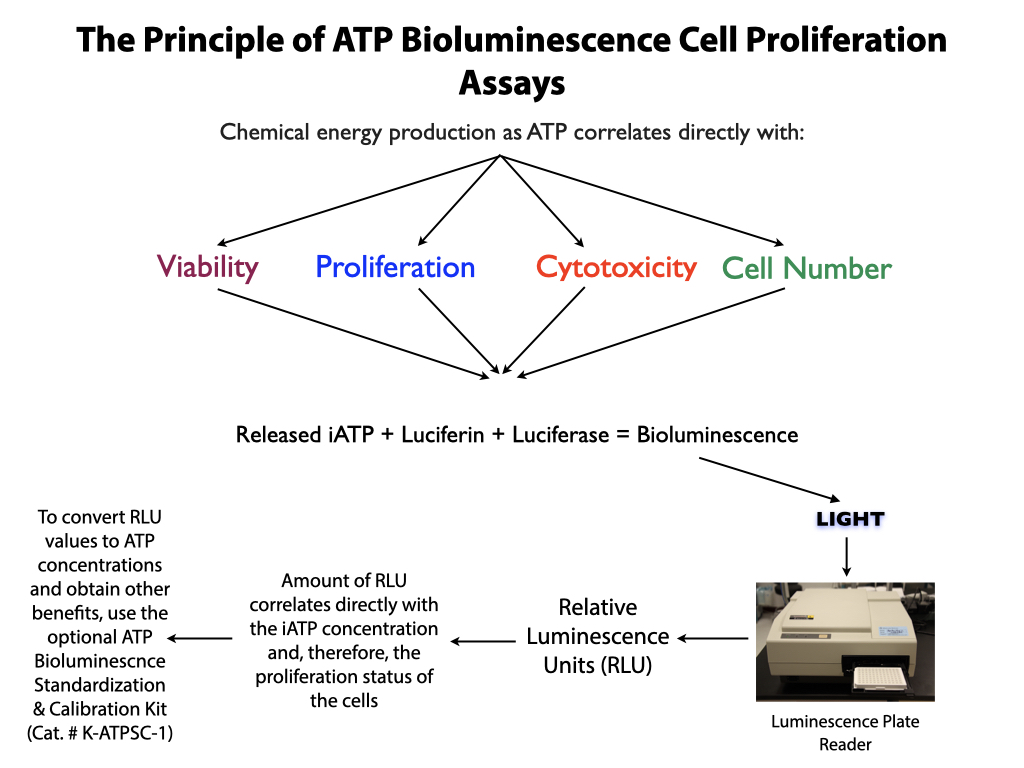
PLEASE NOTE. If HemoGLO™ needs to be standardized and/or validated, if proficiency testing is needed or measurement assurance parameters required, standards and controls are available for purchase separately or use the comprable HALO® Assay Kit.
The table below shows the HemoGLO™ PCA assays that can replace any methlcellulose, colony-forming unit (CFU) reagent from any competitor. PLEASE NOTE that HemoGLO™ PCA incorporates Suspension Expansion Culture™ (SEC™) Technology to replace dispensing error-prone methylcellulose with reagents that use normal pipettes.
| HemoGLO™ PCA Number | HemoGLO™ PCA Cell Population |
Equivalent CFU Cell Population | Equivalent MethoCult™ Reagent |
Growth Factor Cocktail |
|---|---|---|---|---|
| PCA1 | SC-GEM 3 | CFC-GEM 3 | H4434 "Classic" | EPO, GM-CSF, IL-3, SCF |
| PCA2 | SC-GEM 2 | CFC-GEM 2 | H4034 "Optimum" | EPO, GM-CSF, G-CSF, IL-3, SCF |
| PCA3 | P-GM 1 | GM-CFC 1 | H4534 "Classic" | GM-CSF, IL-3, SCF |
| PCA4 | P-GM 2 | GM-CFC 2 | H4035 "Optimum" | GM-CSF, G-CSF, IL-3, SCF |
| PCA5 | SC-GEMM 3 | CFC-GEMM 3 | H4435 "Enriched" | EPO, GM-CSF, G-CSF, IL-3, IL-6, SCF TPO(#) |
| PCA6 | SC-GEMM | CFC-GEMM | Methocult "Express" | Recombinant cytokines EPO |
(#) denotes that the Preferred Cell Systems™ formulation of this product includes TPO that stimulates the production of megakaryopoiesis. MethoCult™ and other competitor formulations of this specific product do not include TPO, do not stimulate the production of megakaryocytes and therefore do not detect the CFC-GEMM stem cell population.
HemoGLO™ PCA can be used for the following tissues:
- Mobilized peripheral blood
- Umbilical cord blood
- Bone marrow
- Purified cell populations for any of the above tissues
The recommended cell purity is a mononuclear cell (MNC) fraction. A total nucleated cell (TNC) fraction is not recommended as this contains high concentrations of cell impurities, such as red blood cells, neutrophils, platelets and other cells that severely underestimate and even inhibit the detection of progenitor cells.
- Standardization of the CFU-GM assay using hematopoietic growth factors. J. Hematotherapy:6:191-192 (1997)
- Development of a novel assay to evaluate the functional potential of umbilical cord blood progenitors. Transfusion. 48:620-628 (2008).
- Potency, Proliferation and Engraftment Potential of Stem Cell Therapeutics: The Relationship between Potency and Clinical Outcome for Hematopoietic Stem Cell Products. J Cell Sci Therapy. (2013).
- Detecting primitive hematopoietic stem cells in total nucleated and mononuclear cell fractions from umbilical cord blood segments and units. J Translational Medicine 13:94 (2015)
- Improving quality and potency testing for umbilical cord blood: A New Perspective. Stem Cells Translational Medicine. 4:967-973 (2015)
- Hematopoietic stem cell potency for cellular therapeutic transplantation. In: Hematopoietic Stem Cells, Ed. Rosana P. Camacho. Published by: InTech Open Access Publisher. ISBN 978-953-307-746-8. (2011).
- Measuring the Potency of a Stem Cell Therapeutic. In: Stem Cell Protocols. Methods in Molecular Biology, 1235, Ed. Rich IN. Published by Human Press (2015).
- Bioluminescence Potency Measurement of Cellular Therapy Products. In: Cellular Therapy: Principles, Methods, and Regulations, 2nd Edition, Eds. Areman EM and Loper K. Published by AABB.
- Present Cord Blood Testing Fails to Determine if the Stem Cells Used for Transplantation are of High Quality and PotencyThe Parents Guide to Cord Blood Foundation
- The Difference Between Stem Cell Viability and Potency: A Short Guide for Parent and Patients. The Parents Guide to Cord Blood Foundation
Please note that the following examples were performed using HALO®. However, since both HemoGLO™ and HALO® incorporate the same technology, the examples given below can also be applied to HemoGLO™.
Click image for larger view
Click image for larger view
Correlation of HemoGLO™ PCA with MethoCult™ CFU Formulations:
Why Methylcellulose CFU Assays are now Obsolete.
Top Diagram; Upper Graph
The upper graph demonstrates a direct correlation of HemoGLO™ Progenitor Cell Assays (PCA) measured on day 5 with the equivalent MethoCult™ formulations for which colony counts were assessed on days 7 and 10. The correlation coefficient (r2) is lower on day 7 than on day 10. This is to be expected, since colonies cannot be differentiated on day 7. Nevertheless, the correlation between the measured ATP concentrations on day 5 signifcantly correlate with the colony counts on days 7 and 10 for each of the different growth factor formulations used (see lower graph). Note also that there is a correlation between the primitive hematopoietic stem cell population SC-GEMM 1, measured on day 5 and MethoCult Express, determined on day 7. Indeed, there is no advanatge to using the more expensive "Express" formulation. These correlations indicate not only that any HemoGLO™ PCA assay can predict the results of the equivalent MethoCult formulation, but all HemoGLO™ PCA assays can replace all of the MethoCult™ CFC assay reagents with far greater accuracy, sensitivity, precision and reproducibility.
There are 2 primary reasons for this. (1) All HemoGLO™ PCA assays were originally derived from the traditional CFU assay. (2) All HemoGLO™ PCA assay measure cell proliferation, which occurs prior to the differentiation of cells allowed in the CFU assay.
Top Diagram; Lower Graph
The lower graph is an extension of the upper graph in that it includes HemoGLO™ PCA5,which is equivalent to using MethoCult H4435 "Enriched". In this graph, the ATP measurements have been extended to 7 days instead of 5 days. The result is a higher correlation coefficient between the two assays. Again any of the CFU assays can be replaced by a HemoGLO™ PCA assay, making the use of methylcellulose CFU assays obsolete.
Bottom Diagram
The bottom diagram shows the correlation between HemoGLO™ PCA formulations and equivalent MethoCult™ CFU formulations for human bone marrow. The correlation coefficients are even greater than for cord blood, indicating the higher variability of the CFU assay when cord blood is used.
These results clearly demonstrate that the methylcellulose CFU assays are now obsolete, since HemoGLO™ PCA predicts and produces far superior results and is significantly more reliable and reproducible.
It should also be noted that similar correlations occur when using HemoFLUOR™ PCA or HemoLIGHT™ PCA instead of HemoGLO™ PCA.
When You Use HemoGLO™ PCA, No Colony Counting is Necessary
Another reason why the CFU assay is obsolete is because HALO® make it unnecessary to count colonies.
Since HemoGLO™ PCA does not use methylcellulose, no colonies are produced. Instead, HemoGLO™ PCA incorporates Suspension Expansion Culture™ (SEC™) Technology; cells are cultured in suspension. This means it is easier, faster and more accurate to setup HALO® cultures because normal pipettes are used. The question always asked is: If no colonies are produced, how do I know whether the cells have differentiated and how do I detect cell differentiation? The answer lies in flow cytometry. The type of cells produced is dependent on the growth factor cocktail used, just as it is in a normal CFU assay. The diagram above show this. If a SC-GEMM culture is setup in HemoGLO™ PCA, you would expect to see the presence of stem cells, erythroid cells, granulocytes and macrophages and megakaryocytes, because that is the definition of the SC-GEMM stem cell population. If the cells are removed from a SC-GEMM culture and analyzed by flow cytometry, these are precisely the cell types that are present in the culture as seen by the blue columns. Similarly, if SC-BFU cultures are setup, you would expect to see a prevalence of CD235a+ (glycophorin-A) cells and that is exactly what you find, together with some granulocytes and macrophages as well as megakaryocytes. In other words, the cells you detect in HemoGLO™ PCA cultures by flow cytometry are the cells you would expect for the growth factor cocktail used. Therefore, waiting for differentiation to occur and enumerating different types of colonies is unnecessary when using HemoGLO™ PCA.
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- HemoGLO™ PCA Master Mix
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Sterile, adhesive foil covers
To learn how to setup and use Progenitor Cell Assays (PCAs), click on the video links below:
How to Perform a Progenitor Cell Assay (PCA) - An Alternative to All Methylcellulose CFU Assays
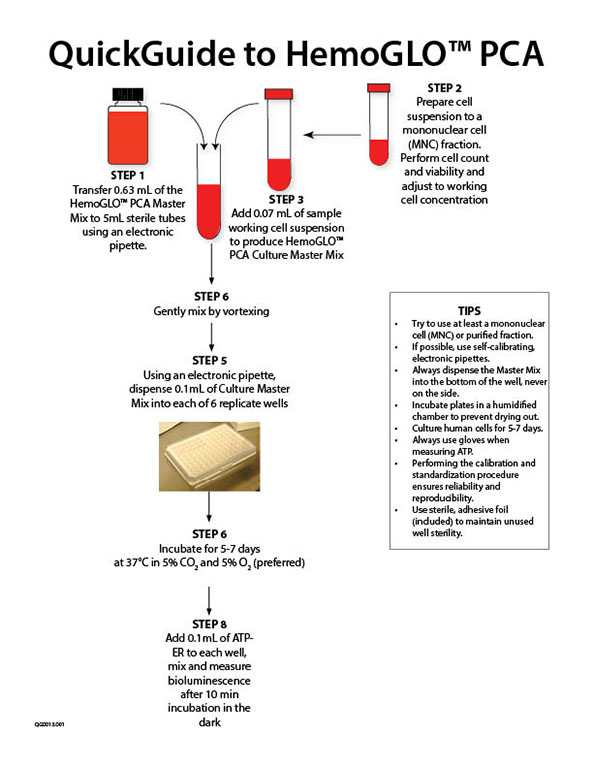
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM