HALO® TE
An In Vitro Standardized and Validated Bioluminescence Assay to Predict Neutrophil, Platelet and/or Erythroid Engraftment
After Stem Cell Transplantation
Buy HALO® TE
Halo® TE
A Standardized and Validated Bioluminescence Assay to Predict Neutrophil, Platelet and/or Erythroid Engraftment
| Description | Species & Tissue | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Lineage-Specific Cells | |||||
| P-BFU 1 | Bone marrow or peripheral blood | Low serum | K2-BTE-1 | 1 Kit | |
| P-GM 1 | Bone marrow or peripheral blood | Low serum | K2-GMTE-1 | 1 Kit | |
| P-Mk 1 | Bone marrow or peripheral blood | Low serum | K2-MkTE-1 | 1 Kit | |
| P-BFU1 + P-GM 1 + P-Mk 1 | Bone marrow or peripheral blood | Low serum | K2-3PTE-3 | 1 Kit | Lineage-Specific Cells |
| B-BFU 1 | Bone marrow or peripheral blood | Serum-Free | K2SF-BTE-1 | 1 Kit | P-GM 1 | Bone marrow or peripheral blood | Serum-Free | K2SF-GMTE-1 | 1 Kit | P-Mk 1 | Bone marrow or peripheral blood | Serum-Free | K2SF-MkTE-1 | 1 Kit | P-BFU 1 + P-GM 1 + P-Mk 1 | Bone marrow or peripheral blood | Serum-Free | K2SF-3PTE-3 | 1 Kit |

Download the Time to Engraftment Assay Flyer
HALO® TE - Uses, Benefits and Characteristics
- No need to wait for >500 neutrophils/µL and 50,000 platelets/µL of blood after transplantation. HALO® TE can predict the production of neutrophils, platelets and/or red blood cells.
- No 14 day cultures. Just a short 5 day incubation that can predict engraftment.
- Perform multiple time prior to actual engraftment. Especially important for cord blood, which requires longer to engraft than bone marrow or mobilized peripheral blood.
- No tedious colony counting required. Cell proliferation data provides rapid, efficient and reliable information.
- Suspension Expansion Culture™ (SEC™) Technology allows high accuracy and precision and rapid dispensing of reagents making the use of methylcellulose obsolete.
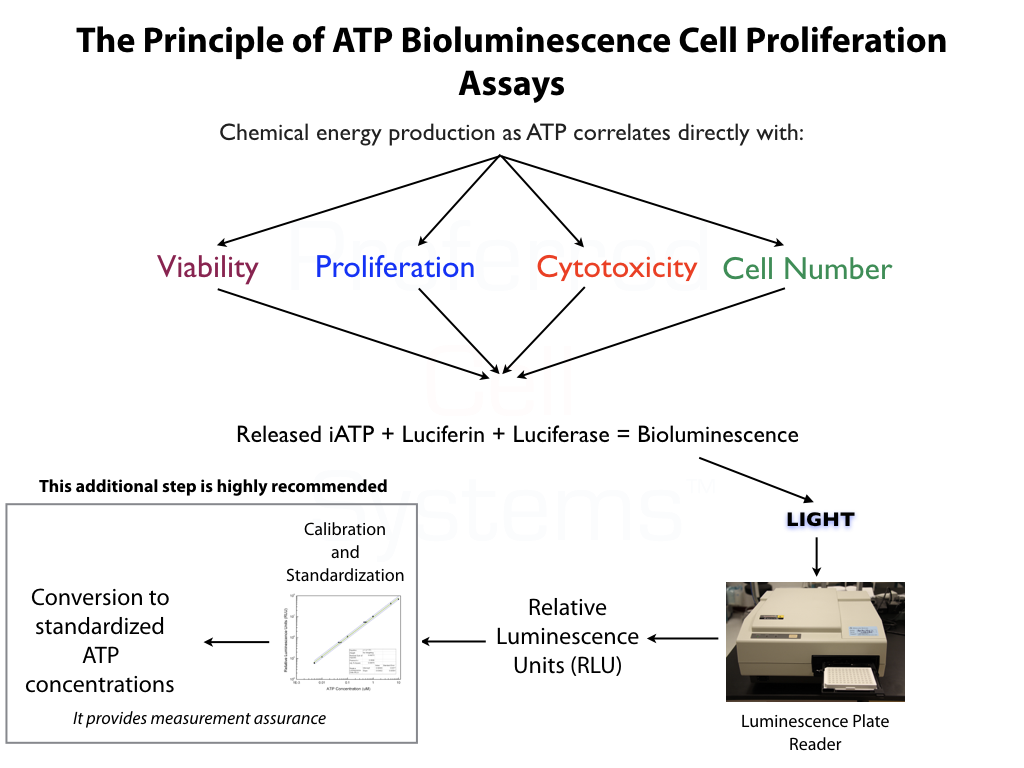
- Incorporates ATP Bioluminescence Technology, the most sensitive and accurate readout available.
- Fully standardized, verified and validated readout according to FDA guidelines.
- Proficiency testing performed and completed during assay standardization procedure. No additional costly and time-consuming proficiency testing required.
- Measurement assurance parameters provide proficiency and trust in your results.
- Correlates with CFU assay; multiplexes with flow cytometry and other readouts.
- 96-well plate format ensures smaller sample and reagent volumes with faster setup.
- Let the plate reader acquire and calculate results in just 5 minutes or less.
- Directly compare potential engraftment of different donor tissues in patients to provide qualified historical data over time.
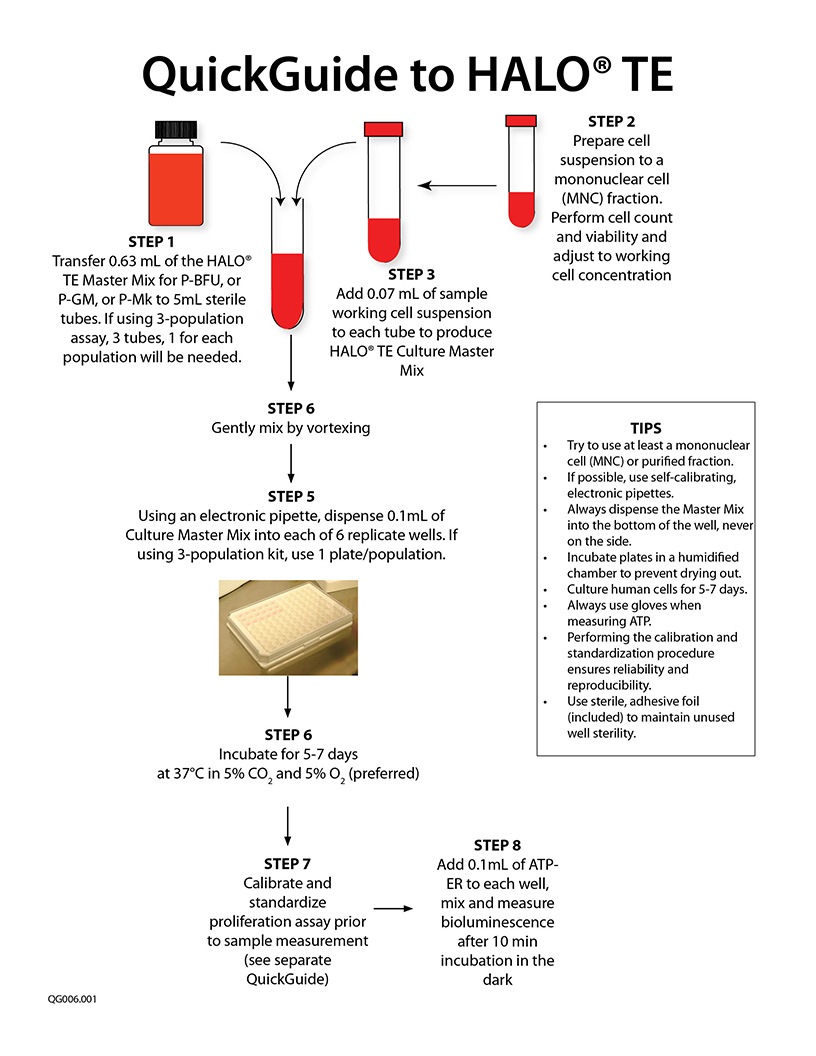
- Includes everything needed to culture and measure lineage-specific or tri-lineage engraftment. Just prepare and add cells.
- Time-efficient and highly cost-effective.
- Easy to learn in just 1 day.
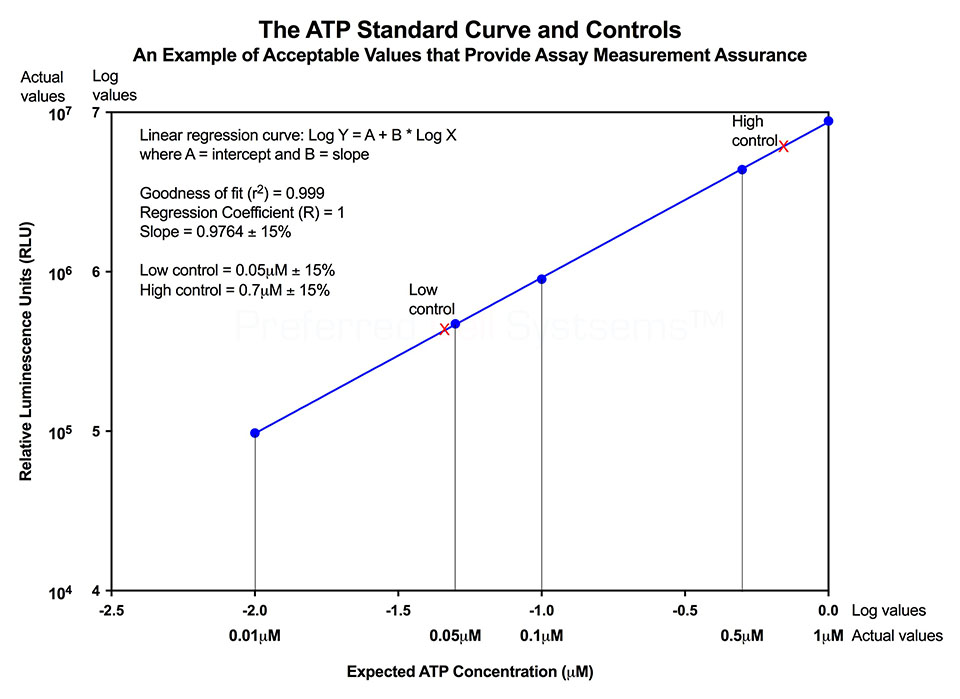
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- P-BFU 1 for erythroid engraftment.
- P-GM 1 for neutrophil engraftment
- P-Mk 1 for platelet engraftment
- P-BFU 1 + P-GM 1 + P Mk 1 for multilineage engraftment
- Umbilical cord blood
- Peripheral blood
- Bone marrow biopsy
Tissues Used for Detection
- Peripheral blood
- Bone marrow biopsy
The recommended cell purity is a mononuclear cell (MNC) fraction. A total nucleated cell (TNC) fraction is not recommended as this contains high concentrations of cell impurities, such as red blood cells, neutrophils, platelets and other cells that severely underestimate and even inhibit the detection of progenitor cells.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- HALO® PT Master Mix (Low serum or serum-free formulations)
- ATP standard
- ATP high and low controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
A specific video tutorial on using the TE assays is not yet ready. Please scroll down and check out the QuickGuide and Technical Manual (if available). Below, are the links to perform the ATP bioluminescence, fluorescence or absorbance readout:
How to Calibrate and Standardize an ATP Bioluminescence Assay - Part 1 (for HALO® TE)
How to Calibrate and Standardize an ATP Bioluminescence Assay - Part 2 (for HALO® TE)
Proficiency Testing for Hematopoietic Cellular Therapy Products
Download the HALO® TE Technical Manual
Download the ATP Optimization Kit Protocol for First-Time Users
Download Luminometer Setup and RLU to ATP Conversion
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM