HemoLIGHT™ TE
The Fast and Easy Absorbance Assay to Predict Neutrophil, Platelet and/or Erythroid Engraftment After Stem Cell Transplantation
Buy HemoLIGHT™ TE
HemoLIGHT™ TE
A Fast and Easy Absorbance Assay to Predict Neutrophil, Platelet and/or Erythroid Engraftment
| Description | Tissue | Formulation | Catalog Number | Quantity | |
|---|---|---|---|---|---|
| Lineage-Specific Cells | |||||
| P-BFU 1 | Bone marrow or peripheral blood | Low serum | K4-BTE-1 | 1 Kit | |
| P-GM1 | Bone marrow or peripheral blood | Low serum | K4-GMTE-1 | 1 Kit | |
| P-Mk 1 | Bone marrow or peripheral blood | Low serum | K4-MkTE-1 | 1 Kit | |
| P-BFU 1 + P-GM 1 + P-Mk 1 | Bone marrow or peripheral blood | Low serum | K4-3PTE-3 | 1 Kit | Lineage-Specific Cells |
| P-BFU 1 | Bone marrow or peripheral blood | Serum-Free | K4SF-GMTE-1 | 1 Kit | P-GM1 | Bone marrow or peripheral blood | Serum-Free | K4SF-MkTE-1 | 1 Kit | P-Mk1 | Bone marrow or peripheral blood | Serum-Free | K4SF-3PTE-3 | 1 Kit | P-BFU1 + P-GM1 + P-Mk1 | Bone marrow or peripheral blood | Serum-Free | K4SF-BTE-1 | 1 Kit |
Instructional Video on How to Use Time to Engraftment (TE) Assays
A specific video tutorial on using the TE assays is not yet ready. Please scroll down and check out the QuickGuide and Technical Manual (if available). Below, is the link to perform the absorbance readout:
How to Perform an Absorbance-Based Readout (for HemoLIGHT TE)
- To rapidly predict and measure the time to neutrophil, erythroid and/or platelet engraftment after hematopoietic stem cell transplantation.
- To promote "Best Practice Criteria Testing."
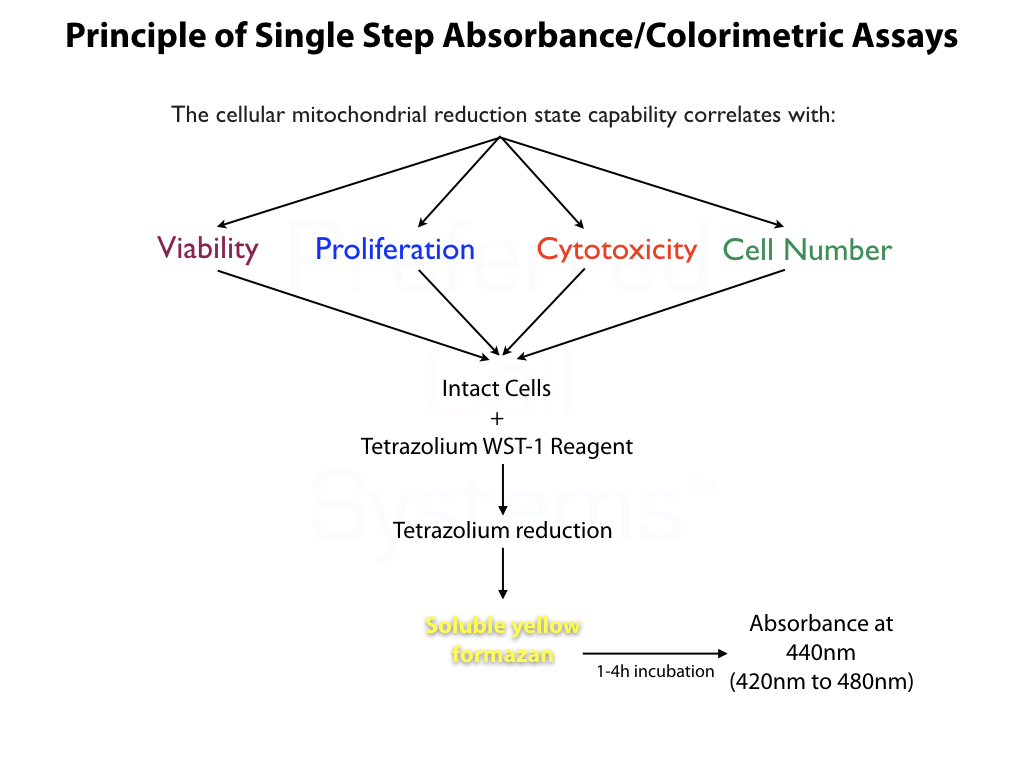
- Measure the ability and amount of GM-CFC, BFU-E and/or Mk-CFC progenitor cell proliferation prior to detecting differentiated cells in a CFU assay.
- Proliferation of progenitor cells can be detected within 5 days.
- Allows for the detection of individual hematopoietic progenitor cell populations or all three simultaneously after transplantation.
- Faster, easier and more accurate to use than any traditional CFU assay, even with automated colony counting.
- Incorporates methylcellulose-free, Suspension Expansion Culture (SEC) Technology to produce rapid, accurate and cost-effective assays.
- Available with low serum or serum-free formulations.
- P-BFU 1 for erythroid engraftment.
- P-GM 1 for neutrophil engraftment
- P-Mk 1 for platelet engraftment
- P-BFU 1 + P-GM 1 + P Mk 1 for multilineage engraftment
- Umbilical cord blood
- Peripheral blood
- Bone marrow biopsy
Tissues Used for Detection
- Peripheral blood
- Bone marrow biopsy
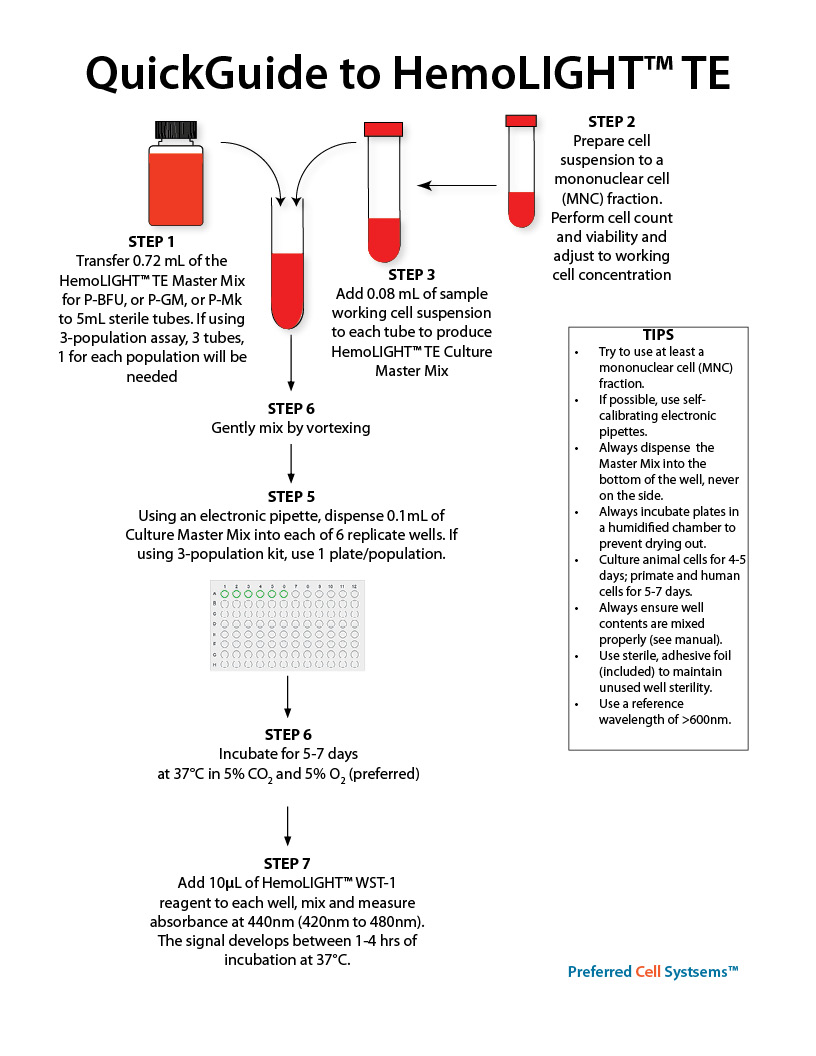
The recommended cell purity is a mononuclear cell (MNC) fraction. A total nucleated cell (TNC) fraction is not recommended as this contains high concentrations of cell impurities, such as red blood cells, neutrophils, platelets and other cells that severely underestimate and even inhibit the detection of progenitor cells.
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- HemoLIGHT™ TE Master Mix(s) as either low serum or serum-free formulations
- WST-1 reagent
- Sterile, clear, 96-well plates
- Sterile, adhesive foil covers