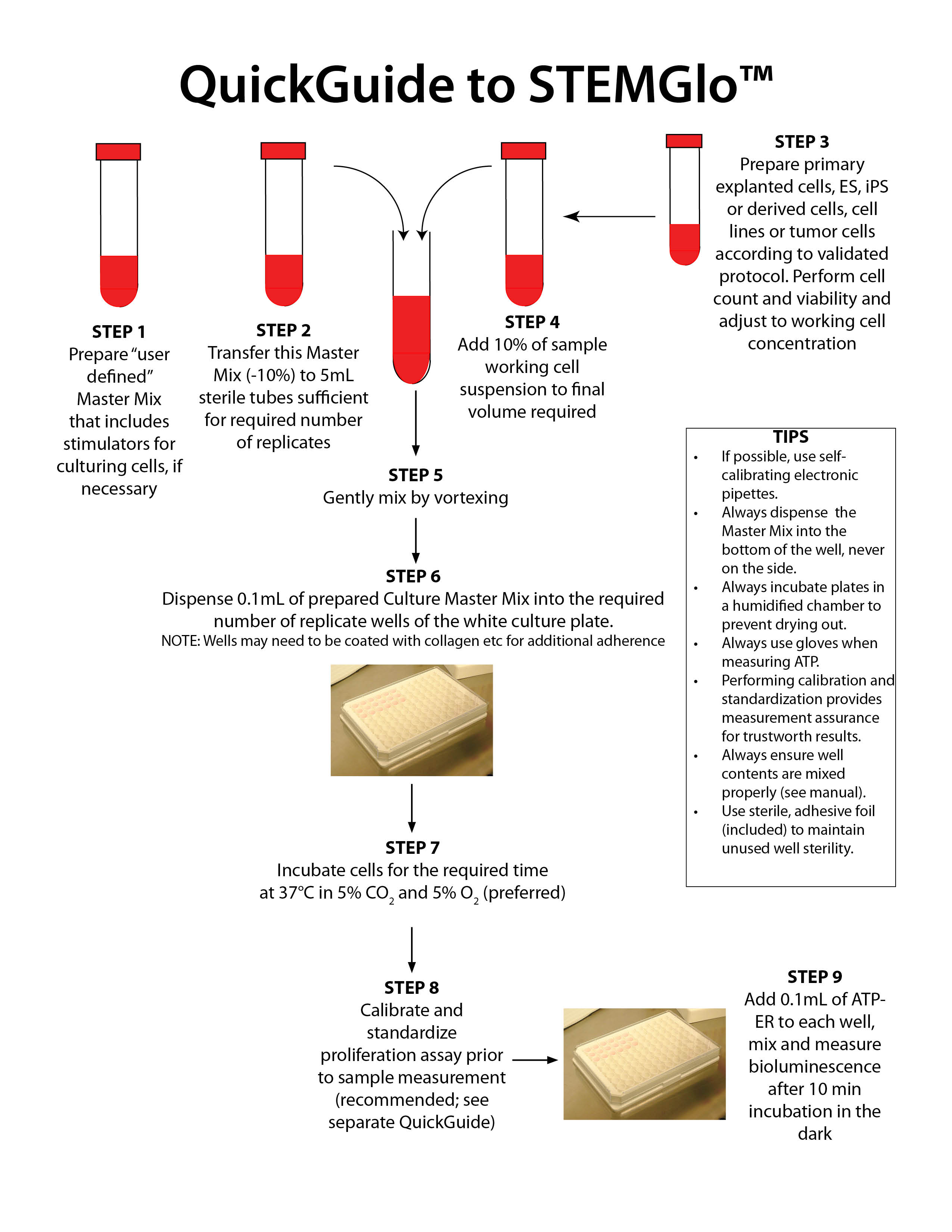
STEMGlo™
An In Vitro Bioluminescence Viability and Functional Assay for ES, iPS and other Transformed Cell Lines
Buy Add-On Standardization Assay Kit
| Description | Catalog Number | Quantity |
|---|---|---|
| An Add-On Kit for Any Bioluminescence "GLO" Assay | K-ATPSC-1 | 1 Kit |
STEMGlo™
The Easy and Rapid In Vitro Research Bioluminescence Assay for Stem Cell Lines
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| ES, iPS and other stem cell lines (Adherent) | Any | User defined | KSG-A-1 | 1 Kit |
| ES, iPS and other stem cell lines (Non-adherent) | Any | User defined | KSG-NA-1 | 1 Kit |
- Study proliferation potential and/or ability of ES, iPS or other stem cell lines.
- Determine the effects of growth factors on stem cell maintenance and growth.
- Study the appearance of intermittent stem cell populations of specific cell lineages.
- Study the kinetics from stem cell proliferation to stem cell differentiation.
- Study the effects of factors required to maintain "stemness".
- Study the effects of CRISPR and other genetic manipulations.
- The most sensitive and accurate assay readout available incorporating proven ATP bioluminescence technology.
- Optional assay standardization using the ATP Bioluminescence Standardization & Calibration Assay Kit (K-ATPSC-1).
- Measurement assurance parameters ensure the assay is working correctly.
- Validated according to FDA bioanalytical method validation guidelines.
- Fast, reliable and reproducible readout with result in 15 minutes for a 96-well plate
- High throughput capability.
- Reliably compare results over time.
- Multiplexing capability allowing the assay to be combined with other endpoints from the same sample.
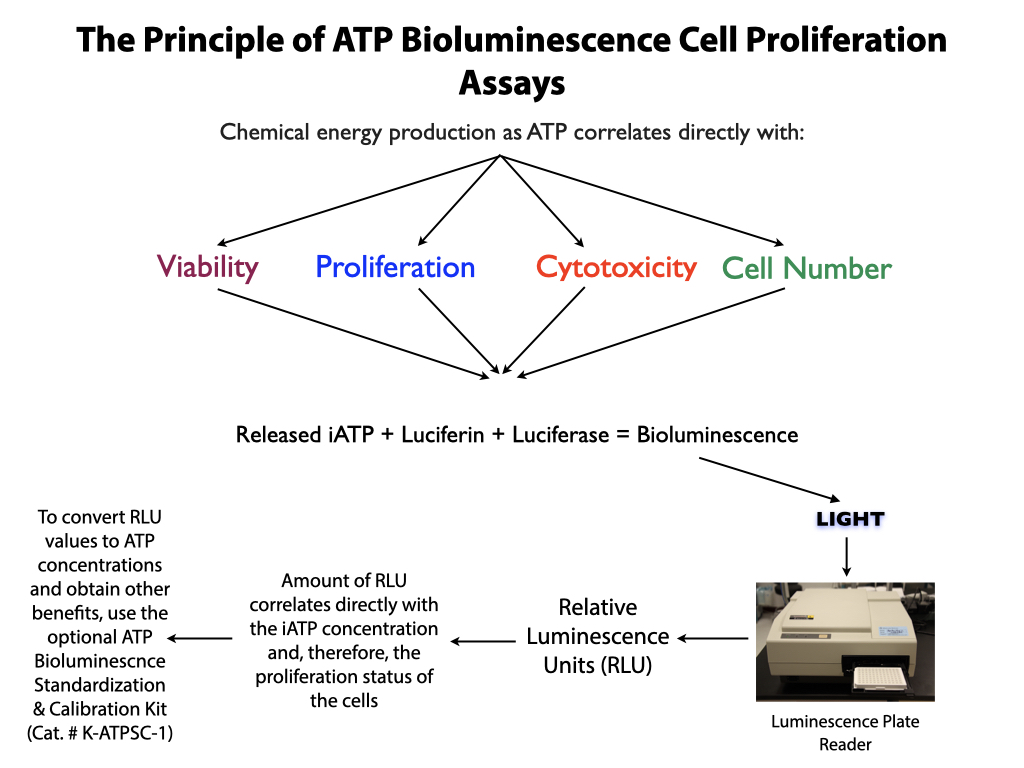
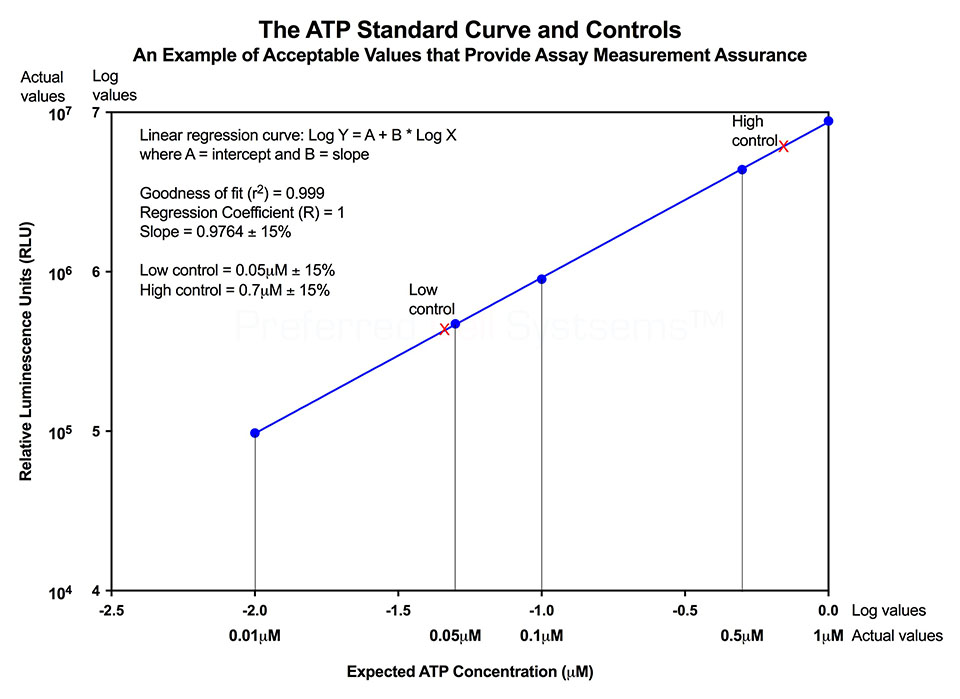
Performing the ATP standard curve and controls is highly recommended, since this allow non-standardized RLU values to be converted to standardized ATP concentrations. The controls are used to calibrate the luminescence plate reader and the ATP standard is used to standardize the assay. The values obtained from the standard curve and controls (see diagram below) are then compared to the measurement assurance parameters that are provided in the instruction manual. If the values obtained are within the ranges provided by the measurement assurance parameters, you, the user, have successfully performed the proficiency testing to ensure that you have not only performed the assay correctly, but that the results you obtain can be trusted.
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
Species and Cell Sources
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.

STEMGlo™ can be used for any ES, iPS or other stem cell line for virtually any species.
Major Equipment Needed
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- ATP Enumeration Reagent
- Sterile, 96-well plates, depending on assay use
- Sterile, adhesive foil covers to maintain sterility of unused wells
Download the STEMGlo™ Manual
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download SDS for ATP Enumeration Reagent
Download SDS for ATP Standards and Controls Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download SDS for ATP Enumeration Reagent
Download SDS for ATP Standards and Controls Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM