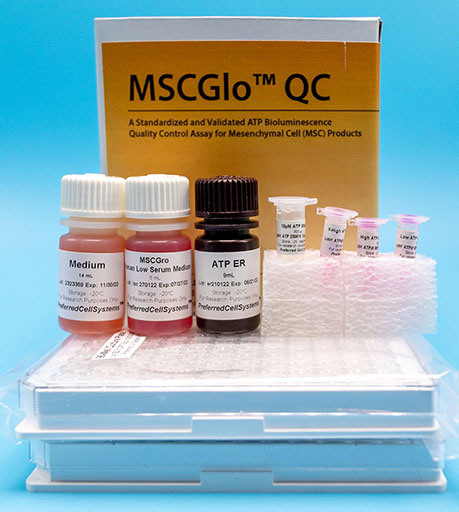
MSCGlo™ QC
A Standardized and Validated In Vitro Bioluminescence Assay to Quality Control Mesenchymal Stromal Cell (MSC) Regenerative Medicine Products During Manufacture
Buy MSCGlo™ QC
MSCGlo™ QC
A Standardized and Validated In Vitro Bioluminescence Assay to Quality Control Mesenchymal Cells (MSC)
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Human | Serum free | KLMC-SFQC-1 | 1 Kit |
Uses
- For quantifying the "quality" of a Mesenchymal Stromal Cell (MSC) product prior to use.
- Determine whether a processing procedure affects MSC "quality" (proliferation ability).
- Determine MSC "quality" pre- and post cryopreservation.
- Determine MSC processing consistency and stability.
- Directly compare MSC lots over time or manufactured under different conditions.
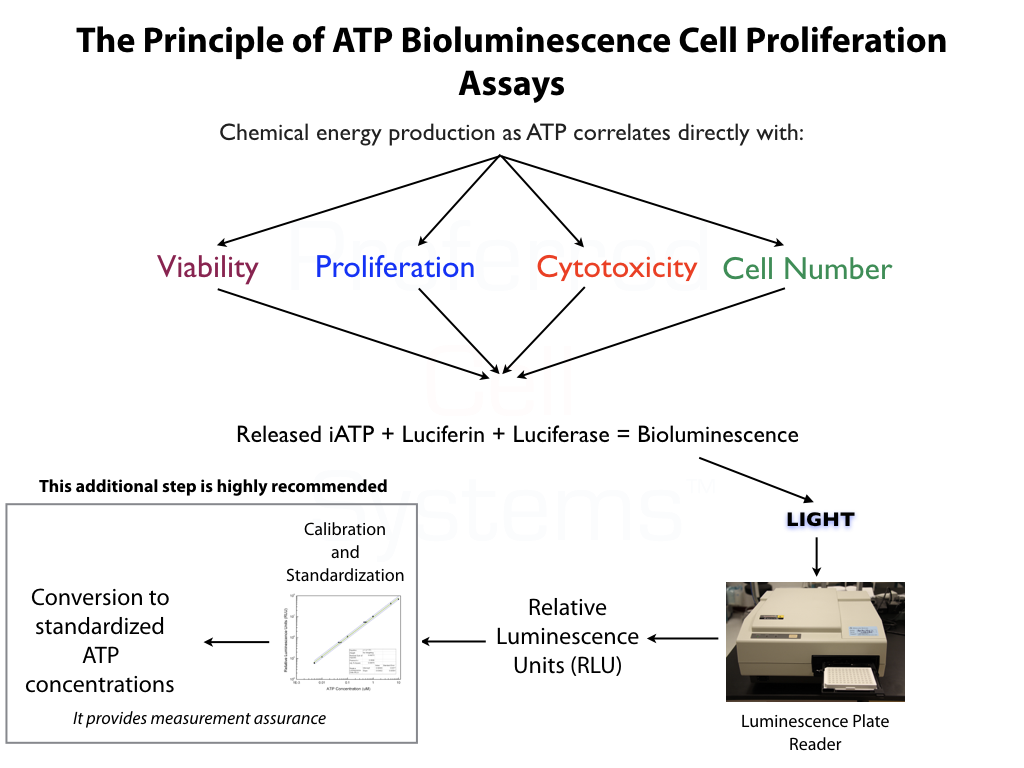
- An ATP bioluminescence, instrument-based and quantitative assay to measure proliferation ability and cellular functionality of clinical-grade MSC/MPC for regenerative medicine.
- Determines population doubling time (PDL), viability and proliferation ability (quality), in a single assay.
- Customize to include different formulations of MSCGro™ Medium.
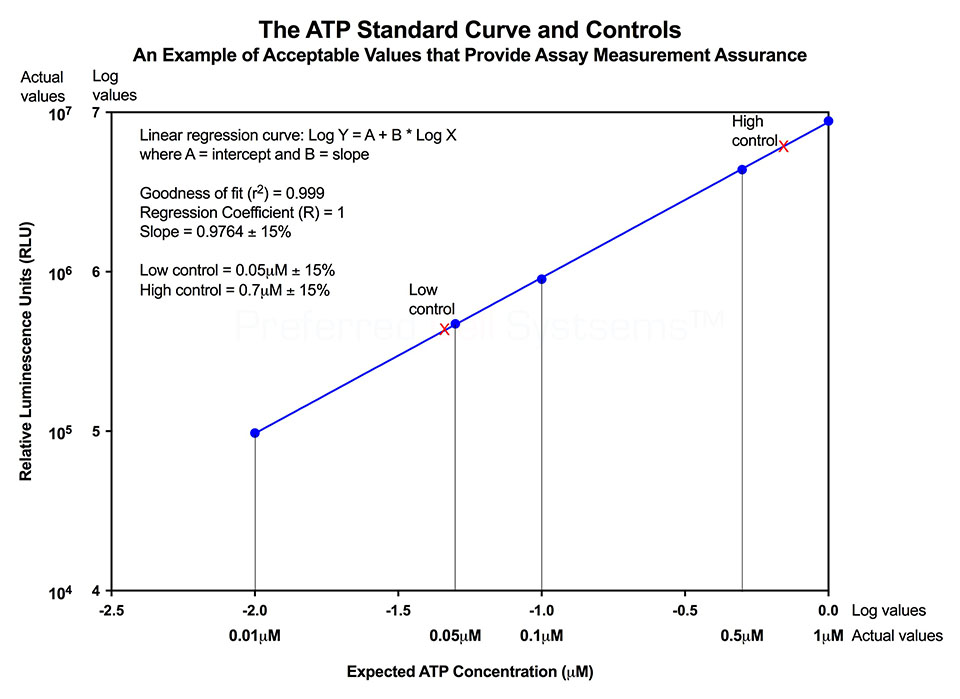
- Fully standardized, incorporating an external standard and controls for assay validation according to FDA regulations.
- Assay can usually be completed in 5-7 days of culture depending on the batch of MSCs.
- Combine/multiplex with flow cytometry to analyze MSC phenotype.
- Easier, more time efficient and far more accurate than the traditional CFU-F assay.
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- MSCGro™ Serum-free, xeno-free complete
- MSCGro™ Humanized, serum-free, complete
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- MSCGro™ Medium of choice
- ATP standard
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
Download the MSCGlo™-QC Manual
Download the ATP Optimization Kit Protocol for First-Time Users
Download the Luminescence Plate Reader Setup and Conversion of RLU to ATP Values Protocol
Download SDS for ATP Enumeration Reagent
Download SDS for ATP Standards and Controls
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM