CellaGlo™-Tox HT
An In Vitro Standardized and Validated ATP Bioluminescence Tumor Cell Toxicity Testing and High-Throughput Screening Platform
Buy Product
Product
Product
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Any tumor cells (adherent) | Any | User defined | KCLG-A-1 | 1 Kit |
| Any tumor cells (non-adherent) | Any | User defined | KCLG-NA-1 | 1 Kit |

Uses of CellaGlo™-Tox HT
- Detecting and measuring potential toxicity to primary tumor or cancer cells for virtually any organ or tissue.
- Specifically designed to screen and test for drugs etc that demonstrate potential toxicity to tumor or cancer cells in vitro.
- High throughput capability during in vitro ADME/Tox screening.
- Allows drug and species ranking of toxicity.
- Incorporates proven, standardized and validated ATP bioluminescence technology, the most sensitive signal detection system available.
- Includes all reagents to calibrate and standardize the assay.
- Measurement assurance parameters included to ensure the assay is working correctly.
- Reliably compare results over time and between drug lots.
- Assay kits include everything needed to perform the readout.
- 96-Well plates included with all kits and available for adherent or non-adherent cells.
- Available as 2- or 4-plate assay kits. Custom sizes also available.
- Designed for multiplexing capability.
- Rapid turnaround.
- Simple and time efficient.
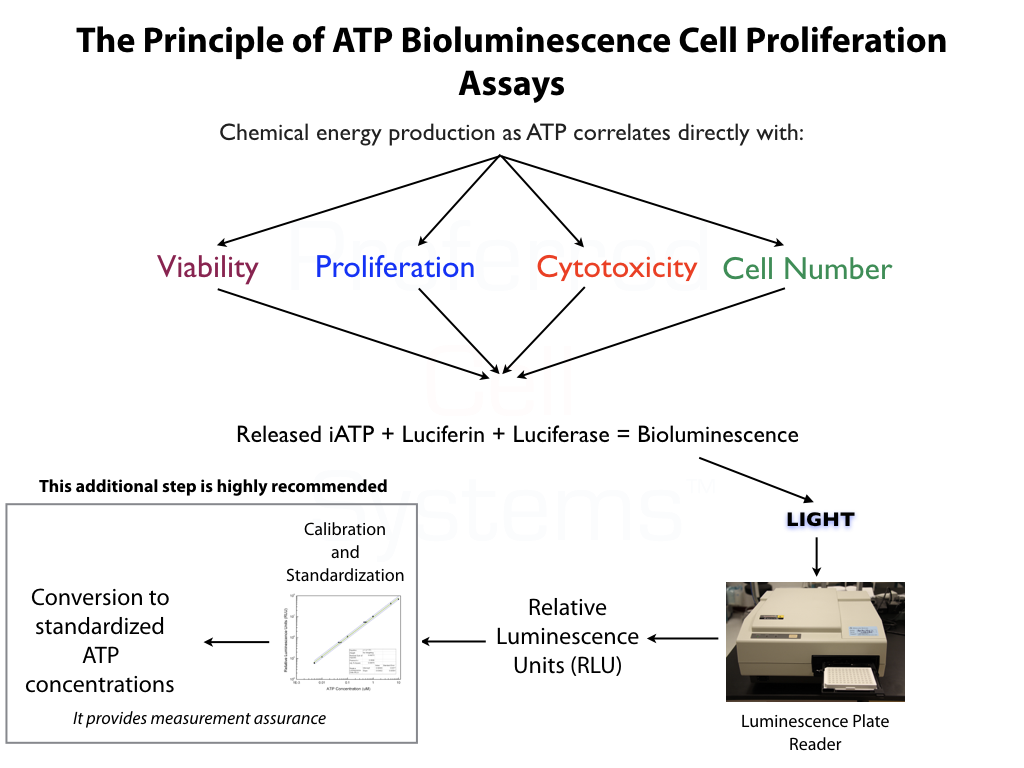
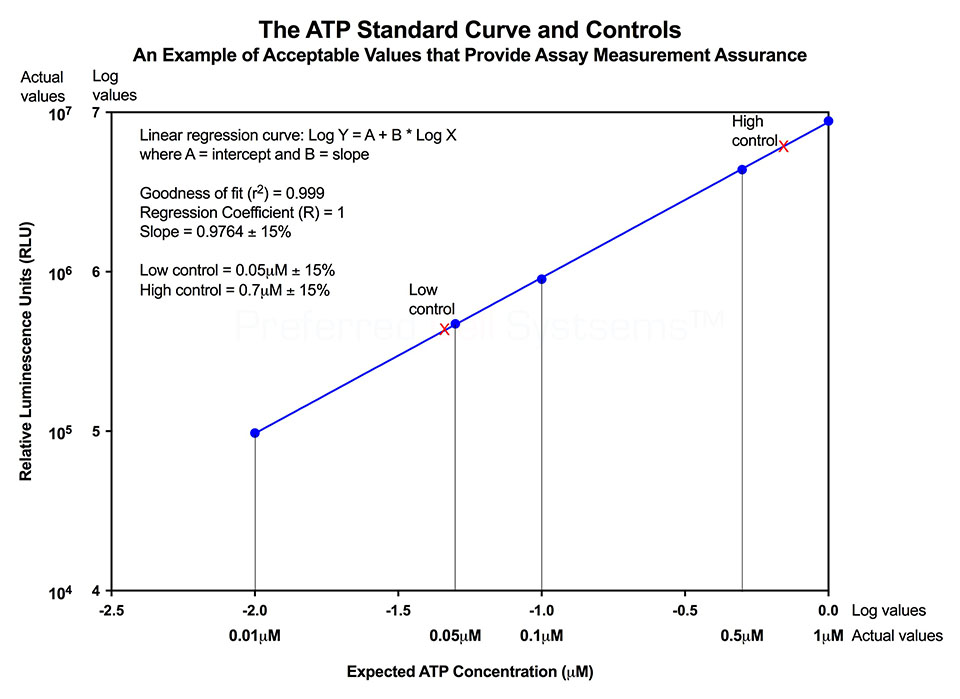
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
CellaGlo™-Tox HT can be used with isolated primary tumor cells from different species. The assay can also be used with tumor cell lines.
CellaGlo™-Tox HT assay kits are include 96-well plates. Upon request, 384-well plates are also available.
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- IMDM base medium for dilution of ATP standard.
- ATP standard.
- ATP controls.
- ATP Enumeration Reagent.
- Sterile, solid white 96-well plates. Available for adherent or non-adherent cells.
- Non-sterile, solid, white 96-well plates.
- Sterile, adhesive foil covers to maintain sterility of unused wells.
Download the CellaGlo™ Technical Manual
Download the ATP Optimization Kit Protocol for First-Time Users
Download Luminometer Setup and RLU to ATP Conversion
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM