MSCGlo™ RS and MSCGlo™ SC-IPS
A Standardized and Validated In Vitro
Bioluminescence Assay Platform to Measure
the Potency Mesenchymal Stromal Cells (MSC)
Used in Regenerative Medicine
Buy MSCGlo™ RS
MSCGlo™ RS
A Standardized and Validated In Vitro Bioluminescence Assay to Establish Potency Reference Standards for Mesenchymal Cells
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Bone marrow-derived MSCs | Serum-free, Humanized | KLMC-SFRS-1BM | 1 Kit |
| Mesenchymal Stem/Stromal Cells | Cord blood-derived MSCs | Serum-free, Humanized | KLMC-SFRS-1CB | 1 Kit |
MSCGlo™ SC-IPS
A Standardized and Validated In Vitro Bioluminescence Assay to Measure Mesenchymal Cell (MSC) Potency
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Human | Serum-free, Humanized | KLMC-SFP-1 | 1 Kit |
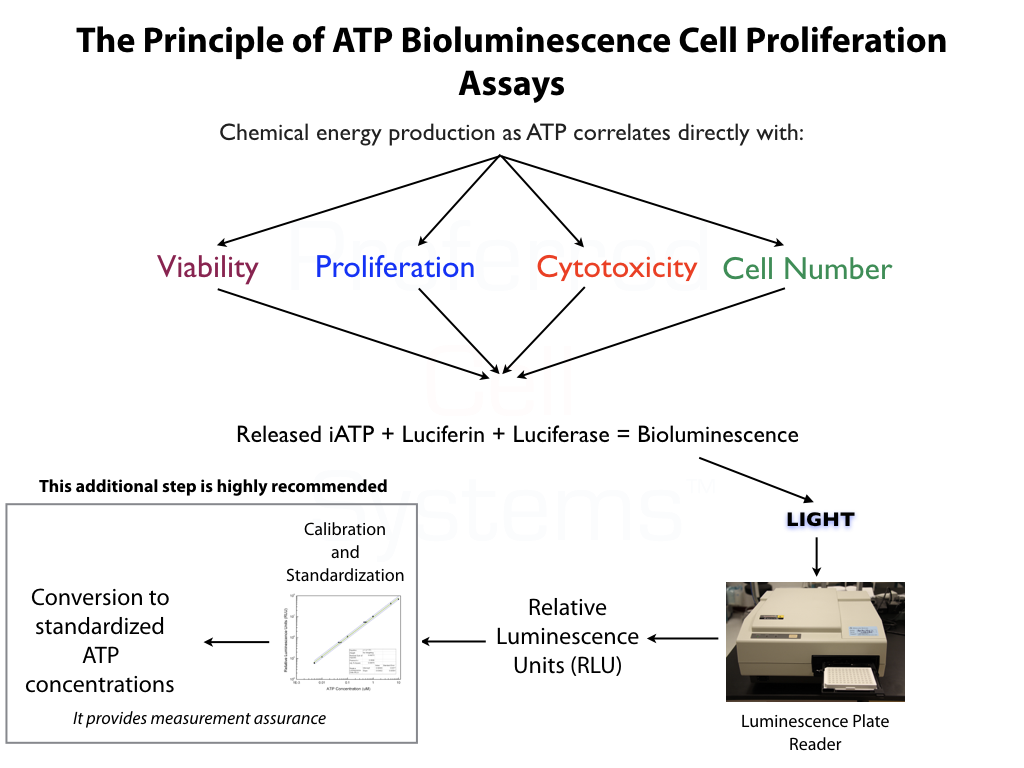
What is Cell Strength or Potency?
- Cell strength or potency is the quantitative measurement of cell proliferation potential. This, in turn, is a measure of cell primitiveness and cell engraftment potential.
- Cell strength predicts a specific response associated with the intended effect. This is usually the capacity of cells to home, seed and begin proliferation at a particular location in the body. Cell potency does not predict whether the cells will actually differentiate and produce functional mature cells.
- To determine identity, purity and strength (potency) of native or expanded Mesenchymal Stromal Cells (MSCs) from any source and multiple species.
- An ATP bioluminescence, reference standard-based potency assay for MSCs manufactured and produced for clinical use.
- Determines population doubling time (PDL), viability, proliferation ability (quality) and proliferation potential (potency) in a single assay.
- MSCGlo™ SC-IPS has been validated according to FDA Bioanalytical Method Validation Guidelines. However, additional validation can be performed since assay kits include standards and controls which, together with the reference standard, allows the assay to be validated in-house. Please see the Instruction Manual for validation parameters.
- Customize the assay with serum-free/xeno-free or humanized MSCGro™ Medium.
- Compliant with FDA regulations for potency assays.
- Uses the "Slope-Ratio Concentration-Response Model" to determine the measure of potency, the potency ratio, standardized in the U.S. Pharmocopeia.
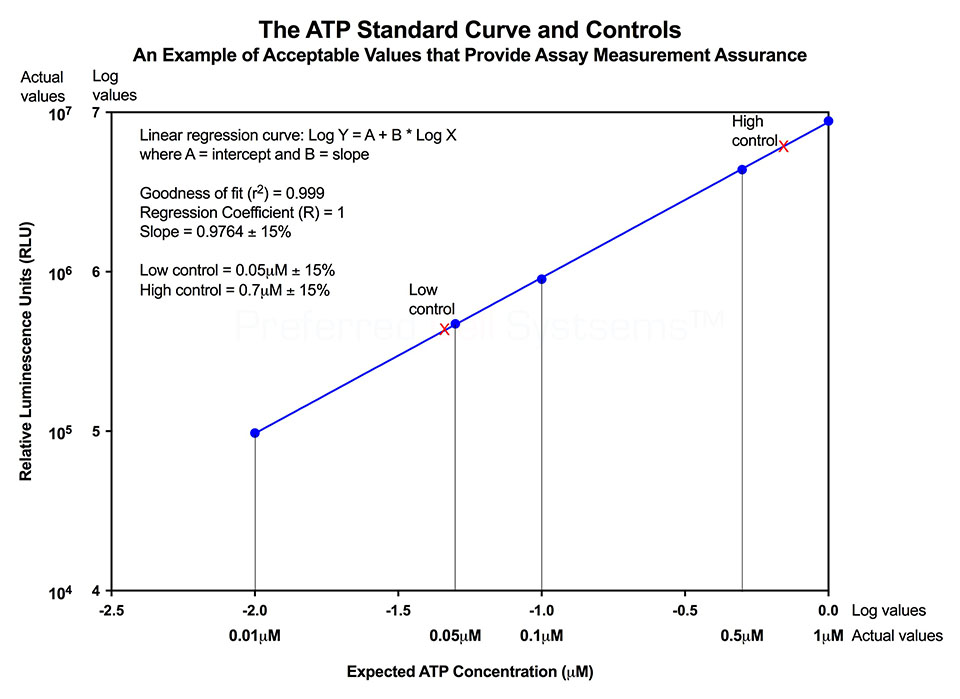
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- MSCGro™ Low serum, complete
- MSCGro™ Serum-free, xeno-free, complete
- MSCGro™ Humanized, serum-free, complete
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
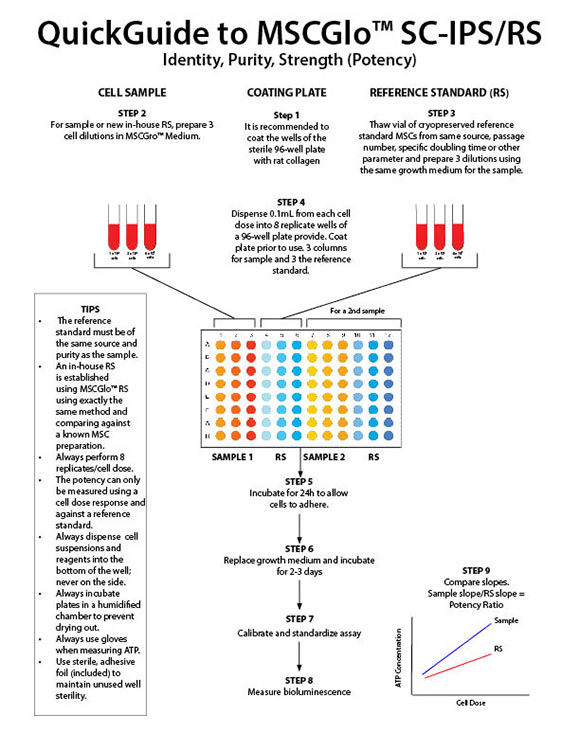
MSCGlo™ RS Kit Contents:
- Vial of cryopreserved reference standard MSCs derived from either bone marrow or umbilical cord blood. For MSCs derived from other tissues, an in-house reference standard can be established using MSCGlo™ Potency
- MSCGro™ Medium of choice
- ATP standards
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plate(s)
- Non-sterile, 96-well plate(s)
- Sterile, adhesive foil covers
MSCGlo™ SC-IPS Kit Contents:
- MSCGro™ Medium of choice
- ATP standard
- ATP controls
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Non-sterile, 96-well plates
- Sterile, adhesive foil covers
Download the MSCGlo™ RS / MSCGlo™ SC-IPS Technical Manual
Download the ATP Optimization Kit Protocol for First-Time Users
Download the Luminescence Plate Reader Setup and Conversion of RLU to ATP Values Protocol
Download SDS for ATP Standards and Controls Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM