MSCGlo™
A Mesenchymal Stromal Cell (MSC) Viability and Proliferation Bioluminescence Assay that Replaces CFU-F
Assays are also available Complete with Cryopreserved MSCs
Buy Add-On Standardization Assay Kit
| Description | Catalog Number | Quantity |
|---|---|---|
| An Add-On Kit for Any Bioluminescence "GLO" Assay | K-ATPSC-1 | 1 Kit |
MSCGlo™
A Mesenchymal Cell (MSC) Bioluminescence Viability and Proliferation Assay that Replaces the CFU-F Assay
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Human | Serum-free, Humanized | KLMC-SF-1 | 1 Kit |
MSCGlo™ Complete
A Mesenchymal Cell (MSC) Bioluminescence Viability and Proliferation Assay Complete with Cryopreserved Cells
| Cell Population | Tissue | Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Mesenchymal Stem/Stromal Cells | Complete with human bone marrow MSC | Serum-free, Humanized | KLMC-SF-BM1 | 1 Kit |
| Mesenchymal Stem/Stromal Cells | Complete with human cord blood MSC | Serum-free, Humanized | KLMC-SF-CB1 | 1 Kit |
Uses
- All research applications involving mesenchymal stem/progenitor cells and cells derived from MSCs.
- Replacement for the colony-forming unit - fibroblast (CFU-F) assay.
- Stromal cell assay applications.
- Combination of MSC proliferation with other readouts, e.g. flow cytometry, gene expression.
- Immunoregulation involving MSC/MPC.
- Self-renewal and differentiation capability applications.
- Clinical and regenerative medicine applications.
- Determine the effects of growth factors/cytokines on MSCs and their differentiation.
- MSC expansion.
- In vivo to in vitro assays.
- MSC gene targeting and expression, e.g. CRISPR-Cas9.
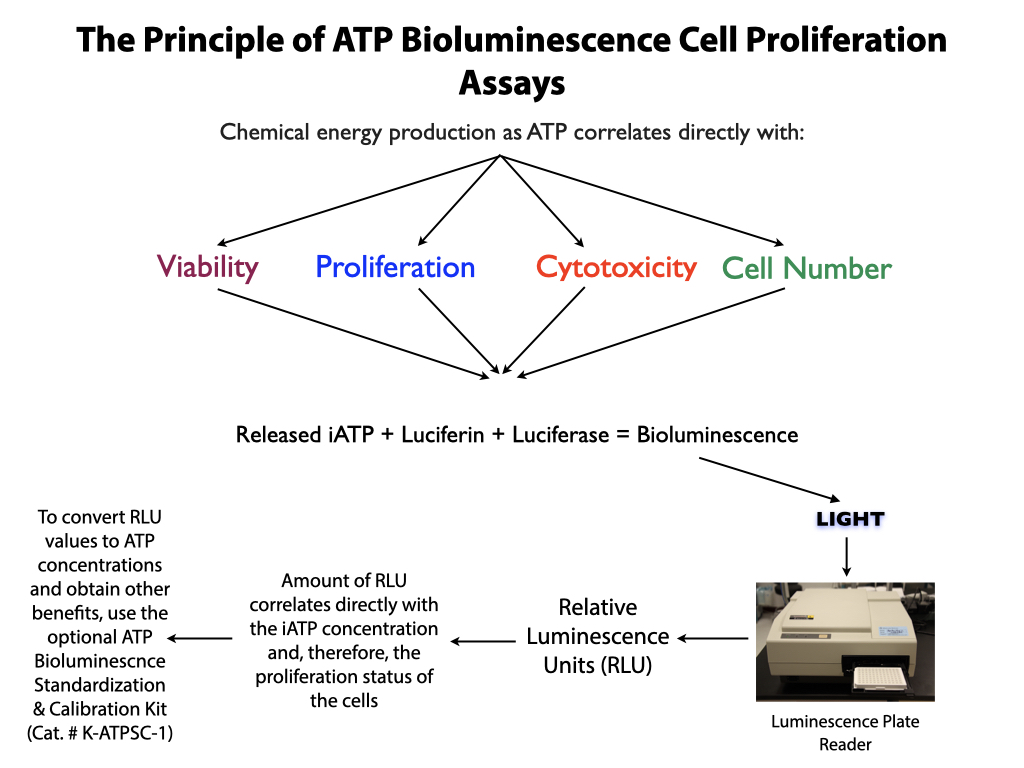
- Uses the chemical energy of intracellular ATP to measure MSC viability, proliferation ability, potential and differentiation.
- Replaces the CFU-F assay with a more reliable, robust, non-subjective, instrument-based assay.
- Allows population doubling time (PDL), viability, proliferation ability and proliferation potential to be measured in a single assay.
- Choose between low serum, serum-free/xeno-free or humanized media included with the assays. Human platelet lysate can also be used.
- Monitor small, medium and large (bulk) MSC/MPC expansion (passaging).
- Assay kits can be customized to include different formulations of MSCGro™ Medium.
- Optional ATP Bioluminescence Standardization & Calibration Kit (Cat. No. K-ATPSC-1) available (see below).
- Multiplex with flow cytometry for phenotypic analysis or gene expression analysis to obtain and correlate information from a single sample.
MSCGlo™-96 Complete
Includes a vial of frozen MSCs derived from either human umbilical cord blood or bone marrow.
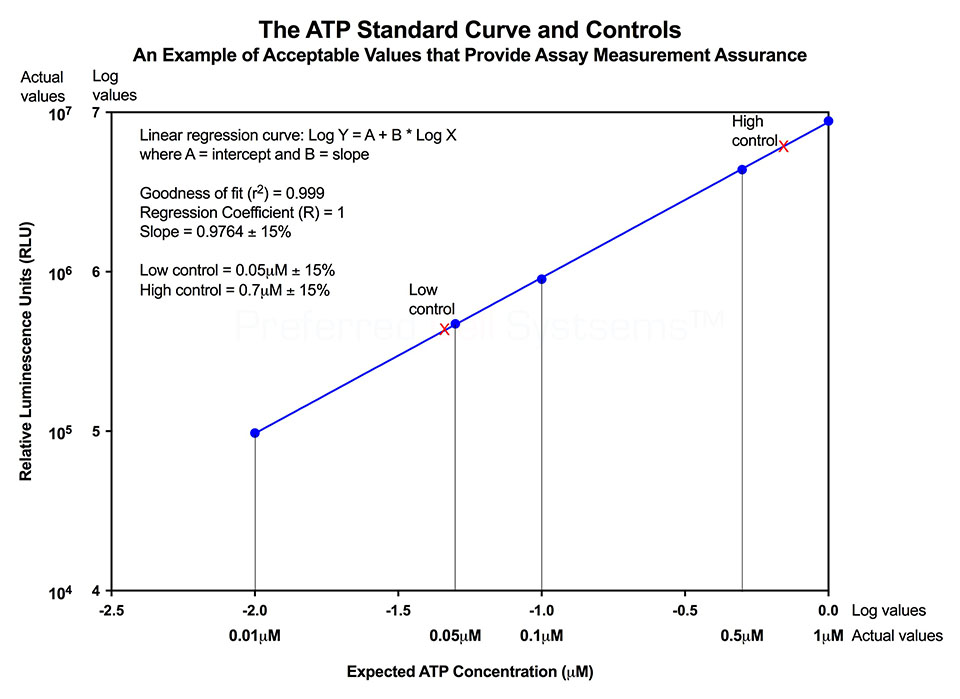
No additional proficiency testing is required if the calibration and standardization procedure is performed. The values you obtain from each calibration and standardization can be logged and used for certification that the assay has been performed correctly and that the results are trustworthy.
- Human
- Non-human primate
- Rat
- Mouse
- Dog
- Horse
MSCGro™ Media Available
- Low serum, complete
- Serum-free, xeno-free, complete
- Humanized, serum-free, complete (for human cells only)
Mesenchymal Stem/Stromal Cells Available (all human)
- Bone marrow
- Umbilical cord blood-derived MSCs
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- MSCGro™ Medium
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Sterile, adhesive foil covers
Kit Contents for MSCGlo™ Complete
- 1 Vial of cryopreserved human umbilical cord blood or bone marrow MSCs
- MSCGro™ Medium
- ATP Enumeration Reagent
- Sterile, 96-well plates
- Sterile, adhesive foil covers
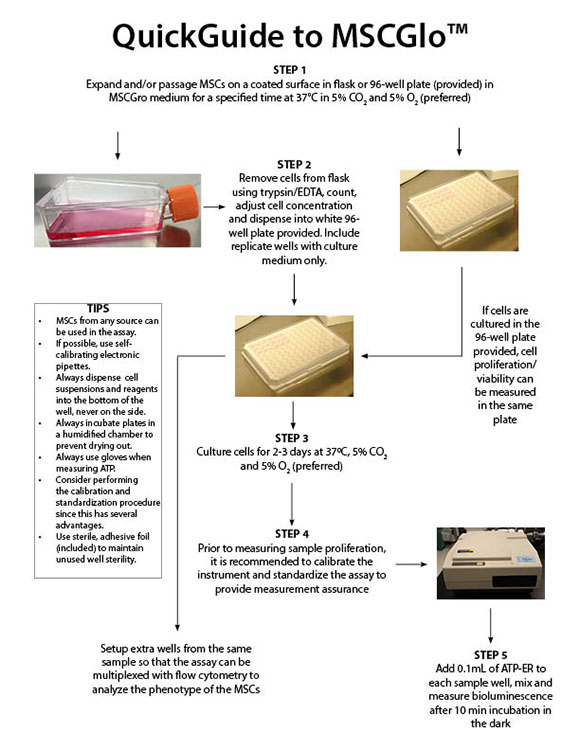
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download SDS for ATP Enumeration Reagent
Download SDS for ATP Standards and Controls Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM