HepatoGLO™
An In Vitro Bioluminescence Assay for Hepatocyte Viability and Function
Buy Add-On Standardization Assay Kit
| Description | Catalog Number | Quantity |
|---|---|---|
| An Add-On Kit for Any Bioluminescence "GLO" Assay | K-ATPSC-1 | 1 Kit |
HepatoGLO™
A Standardized and Validated Vitro Bioluminescence Hepatotoxicity Assay Platform
| Cell Population | Species | Medium Formulation | Catalog Number | Quantity |
|---|---|---|---|---|
| Hepatocytes (adherent) | Any | User defined | KHG-A-1 | 1 Kit |
| Hepatocytes (non-adherent) | Any | User defined | KHG-NA-1 | 1 Kit |
Uses of HepatoGlo™
- Determine viability of hepatocyte cultures.
- Measure hepatocyte life span in culture.
- Study hepatocyte stem cells.
- Determine hepatocyte mitochondrial dysfunction.
- Study liver cell interactions.
- Determine the response of hepatocytes to different agents.
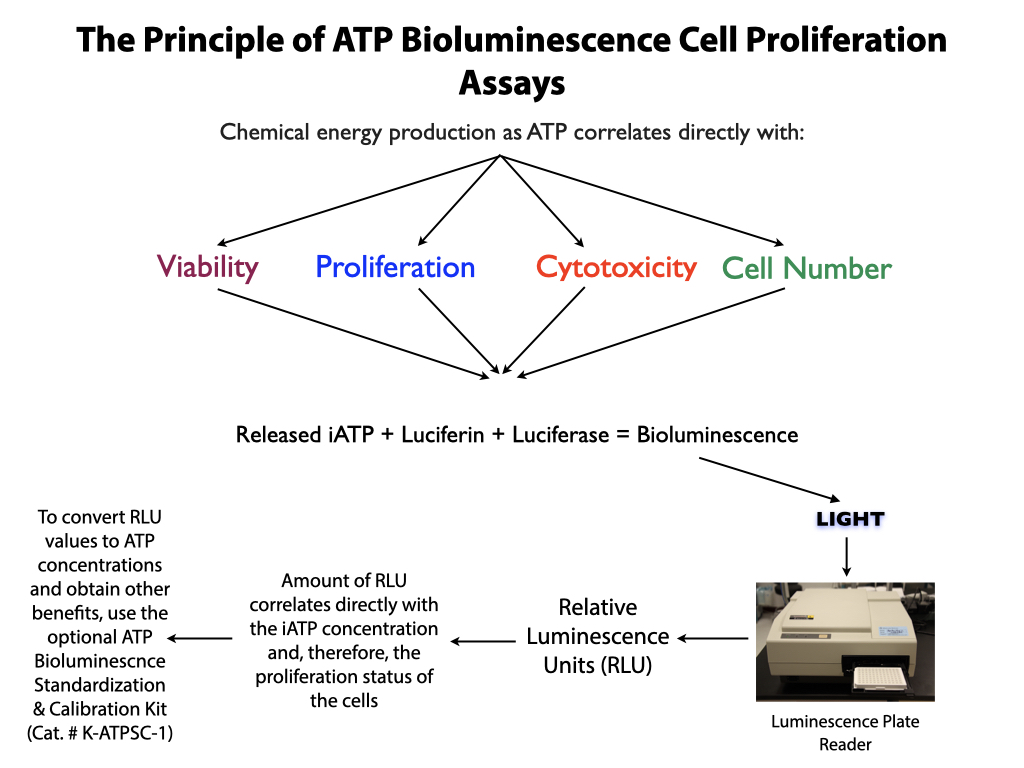
- The most sensitive and accurate assay readout available incorporating proven ATP bioluminescence technology.
- Optional standardization available using the ATP Bioluminescence Standardization & Calibration Kit (K-ATPSC-1).
- Measurement assurance parameters to ensure the assay is working correctly.
- Validated according to FDA bioanalytical method validation guidelines.
- Fast, reliable and reproducible readout with result in 15 minutes for a 96-well plate
- High throughput capability.
- Reliably compare results over time.
- Multiplexing capability allowing the assay to be combined with other endpoints from the same sample.
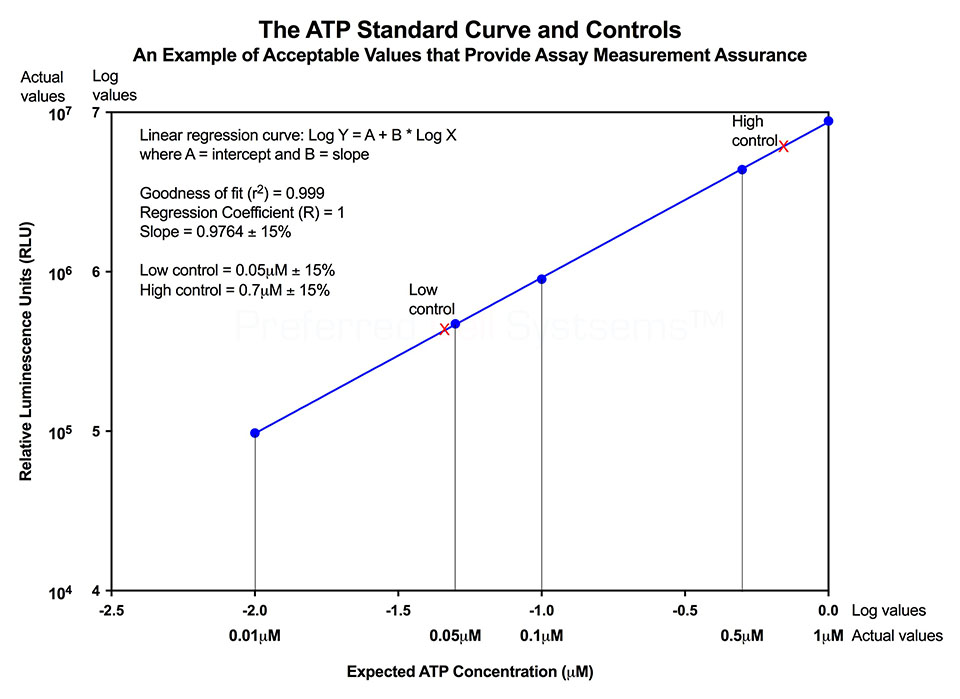
Performing the ATP standard curve and controls is highly recommended since it allows conversion of non-standardized RLU values into standardized ATP concentrations. The controls are used to calibrate the instrument and the ATP standard is used to standardize the assay. The values obtained provide the measurement assurance parameters that allow you, the user, to ensure that the results obtained can be trusted.
An example of an ATP standard curve and control results are shown in the diagram below and actual values for the kit being used are given in the Technical Manual. If the values obtained are within the ranges given in the Technical Manual, the user can process the samples knowing that the results will be trustworthy.
Species and Cell Sources
An example of an ATP standard curve and control results are shown in the diagram below and actual values for the kit being used are given in the Technical Manual. If the values obtained are within the ranges given in the Technical Manual, the user can process the samples knowing that the results will be trustworthy.

HepatoGlo™ can be used for multiple species and different sources of hepatocytes (e.g. fresh, cryopreserved, male, female, mixed and hepatocyte cell lines such as HepG2).
For Research Use Only. Not for clinical diagnostic use.
Luminescence or multimode plate reader with "glow" luminescence measuring capability.
- ATP Enumeration Reagent
- Sterile, white, 96-well plates for hepatocyte culture.
- Sterile, adhesive foil covers to maintain sterility of unused wells
PLEASE NOTE. The investigator has the flexibility to culture hepatocytes using their own hepatocyte growth and/or maintenance medium. However, HepatoGro™ medium can be purchased separately from Preferred Cell Systems™.
Please visit the Instructional Videos page
Download the HepatoGlo™ Technical Manual
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM
Download the HepatoGlo™ Technical Manual
Download the ATP Bioluminescence Standardization & Calibration Kit Manual
Download Certificate of Analysis (CoAs) for ATP Enumeration Reagent (ATP-ER)
Download Certificate of Analysis for ATP Stanadrds
Download Certificate of Analysis for ATP Controls
Download Certificate of Analysis of ATP Reconstitution Reagent
Download Certificate of Analysis for Sterile 96-Well Plates
Download Certificate of Analysis for Non-Sterile, 96-Well Plates
Download Certificate of Analysis for Sealing Films
Download Certificate of Analysis for IMDM